| CPC A61K 31/192 (2013.01) [A61K 9/0073 (2013.01); A61K 31/201 (2013.01); A61K 31/216 (2013.01); A61K 31/343 (2013.01); A61K 31/353 (2013.01); A61K 31/47 (2013.01); A61K 31/4704 (2013.01); Y02A 50/30 (2018.01)] | 23 Claims |
|
1. A method of treating an autoimmune gastrointestinal disorder, wherein the autoimmune gastrointestinal disorder is an inflammatory bowel disease or celiac disease, the method comprising elevating Treg cell numbers and suppressing Th17 cell numbers in an individual having the autoimmune gastrointestinal disorder by administering a therapeutically effective amount of a retinoid X receptor (RXR) agonist having the structure of formula XII:
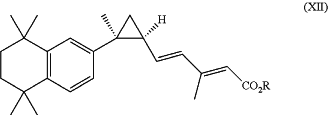 or a pharmaceutically acceptable salt thereof;
wherein R is H or lower alkyl of 1 to 6 carbons;
wherein the therapeutically effective amount is about 0.001 mg/kg/day to about 100 mg/kg/day;
wherein the inflammatory bowel disease is ulcerative colitis or Crohn's disease; and
whereby the balance between Treg and Th17 cell development is modulated to restrain autoimmunity.
|