| CPC C07C 1/22 (2013.01) [B01J 19/0013 (2013.01); B01J 19/245 (2013.01); B01J 23/6484 (2013.01); B01J 23/882 (2013.01); B01J 23/883 (2013.01); B01J 23/8885 (2013.01); B01J 2219/0004 (2013.01); B01J 2219/00051 (2013.01); C07C 2523/648 (2013.01); C07C 2523/882 (2013.01); C07C 2523/883 (2013.01); C07C 2523/888 (2013.01)] | 19 Claims |
|
1. A process for removing hydroxyl groups from phenolic compounds, the process comprising:
providing in a first reactor at least one hydrodeoxygenation catalyst containing sulphided NiMo, CoMo or NiW;
providing a feedstock containing phenolic compounds;
carrying out hydrodeoxygenation by contacting the feedstock with the hydrodeoxygenation catalyst to obtain a phenolic hydrocarbon feedstock;
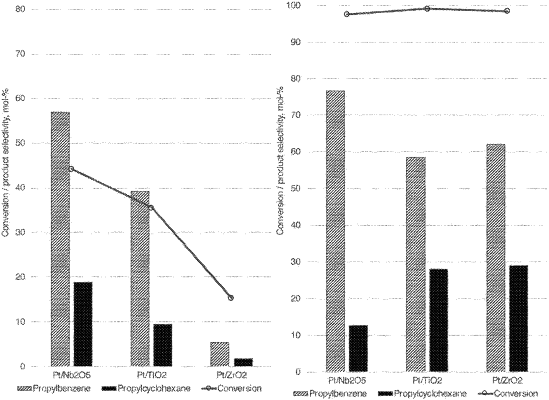
providing in a second reactor at least one direct deoxygenation catalyst containing Pt/Nb2O5;
forming a mixture by feeding into the second reactor the phenolic hydrocarbon feedstock and hydrogen gas; and
carrying out direct deoxygenation to the phenolic hydrocarbon feedstock to obtain hydrocarbons in the second reactor with the hydrogen gas included in the formed mixture during the direct deoxygenation, wherein the direct deoxygenation directly cleaves C—O bonds by hydrogenolysis and at least 50 mol-% of the hydroxyl groups originally bonded to phenolic compounds are removed while the aromaticity of the hydrocarbon is preserved.
|