| CPC C02F 1/725 (2013.01) [C02F 1/722 (2013.01); C02F 1/76 (2013.01); B01J 23/755 (2013.01); C02F 2101/18 (2013.01); C02F 2209/06 (2013.01)] | 6 Claims |
|
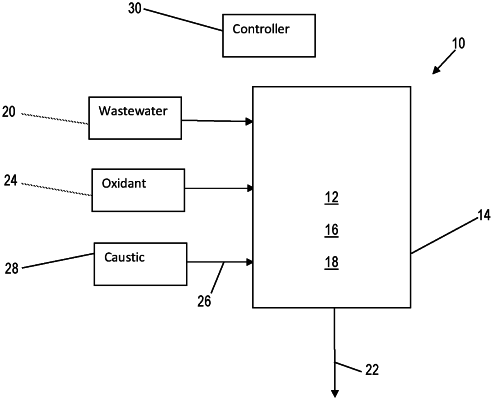
1. A process for selective removal of cyanide from a wastewater (12) comprising both cyanide and chemical oxygen demand (COD), the process comprising:
contacting the wastewater (12) with an amount of an oxidant (16) in the presence of a nickel-based oxide catalyst (18) to selectively remove an amount of the cyanide from the wastewater (12) in a vessel (14); and
maintaining a pH of 8 or more during the contacting of the wastewater (12) with the oxidant (16) and the nickel-based oxide catalyst (18),
wherein maintaining the pH is done via the addition of a caustic (26) to the vessel (14),
wherein pH sensors are associated with the vessel (14) to monitor the pH within the vessel (14), and
wherein a controller (30) is in electrical communication with the pH sensors and is in electrical communication with a source (28) of the caustic (26) for controlling an amount of the caustic delivered to the vessel (14).
|