| CPC A61K 31/12 (2013.01) [A61K 9/127 (2013.01); A61K 31/145 (2013.01); A61K 31/16 (2013.01); A61K 31/355 (2013.01); A61K 31/375 (2013.01); A61K 31/385 (2013.01); A61K 31/7048 (2013.01); A61K 47/10 (2013.01); A61K 47/24 (2013.01); A61K 47/34 (2013.01)] | 17 Claims |
|
1. A pharmaceutical composition comprising:
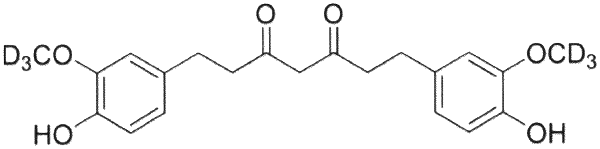
a) tetrahydrocurcumin (THCu) according to the formula:
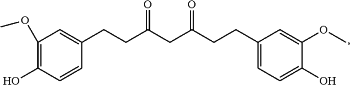 and
b) a pharmaceutically acceptable excipient, wherein the pharmaceutical formulation lacks detectable amounts of hexahydrocurcumin (HHC) as indicated by no peak with a relative retention time of 7.14-7.15 while exhibiting a peak associated with THCu at 9.07 for THCu as determined by gas chromatography (GC).
|