| CPC C07D 471/04 (2013.01) [A61P 11/00 (2018.01); A61P 35/00 (2018.01); C07D 401/12 (2013.01); C07D 405/12 (2013.01); C07D 405/14 (2013.01); C07D 413/14 (2013.01); C07D 417/10 (2013.01); C07D 417/14 (2013.01); C07D 487/10 (2013.01); C07D 513/04 (2013.01); C07D 519/00 (2013.01); C07B 2200/05 (2013.01)] | 40 Claims |
|
1. A compound having the structure:
 wherein
A is
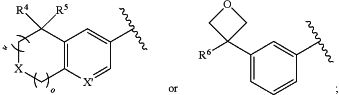 m is 0, 1, 2, or 3;
n is 1 or 2;
o is 0 or 1;
X is O, CH2, or NR7;
X′ is N, CH, or CRX, wherein RX is a halogen;
B is an optionally substituted 6-membered monocyclic heteroarylene or an optionally substituted 9- or 10-membered bicyclic heteroarylene;
L is a covalent bond, optionally substituted C1-C3 alkylene, C2 alkynylene, optionally substituted C2 alkenylene, optionally substituted C2-C3 heteroalkylene, optionally substituted C3-C5 cycloalkylene, or optionally substituted 4- to 10-membered heterocyclylene;
C is optionally substituted 3- to 10-membered cycloalkyl; optionally substituted 6- to 10-membered aryl; optionally substituted 5- to 10-membered heteroaryl; or optionally substituted 5- to 10-membered heterocyclyl;
R1 and R7 are, independently, hydrogen or optionally substituted C1-C6 alkyl;
each R2 and R3 is independently, hydrogen, optionally substituted C1-C6 alkyl; or
optionally substituted C1-C6 heteroalkyl;
R4 is cyano, fluoro, hydroxy, or —CH2OH;
R5 is C1-C3 alkyl optionally substituted with one, two, three, four, five, six, or seven fluoro groups; and
R6 is C1-C3 alkyl optionally substituted with one, two, three, four, five, six, or seven fluoro groups,
or a pharmaceutically acceptable salt thereof.
|