| CPC C07D 401/14 (2013.01) [A61P 35/02 (2018.01); C07D 417/14 (2013.01); C07D 471/04 (2013.01); C07D 471/10 (2013.01); C07D 487/04 (2013.01); C07D 495/14 (2013.01); C07D 498/04 (2013.01)] | 20 Claims |
|
1. A compound having the chemical structure:
CLM-L-PTM,
or a pharmaceutically acceptable salt thereof,
wherein the L is a chemical linking moiety covalently linking the CLM and the PTM, and wherein:
the PTM is
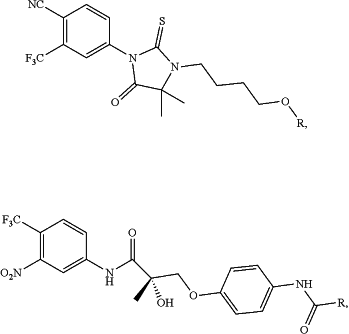  wherein R designates the site of attachment to L; and
the CLM is selected from the group consisting of:
 wherein
R3 is H, C1-C3 alkyl, or C1-C3 alkoxyl;
R4 is H or C1-C3 alkyl;
R5 is H or C1-C3 alkyl;
Q1, Q2, Q3, and Q4 are each independently a carbon substituted with a R′;
R′ is H, halogen, C1-C3 alkyl, or C1-C3 alkoxyl; and
Q5 is N; wherein the CLM is covalently joined to L via R3, R4, R5, R′, Q1, Q2, Q3, or Q4.
|