| CPC A61K 31/517 (2013.01) [A61K 31/4985 (2013.01); A61K 39/39558 (2013.01); A61P 35/00 (2018.01); C07D 403/04 (2013.01); C07D 471/04 (2013.01)] | 4 Claims |
|
1. A method of treating cancer in a subject in need thereof, comprising administering to the subject a therapeutically effective amount of a compound, wherein the compound is
                                          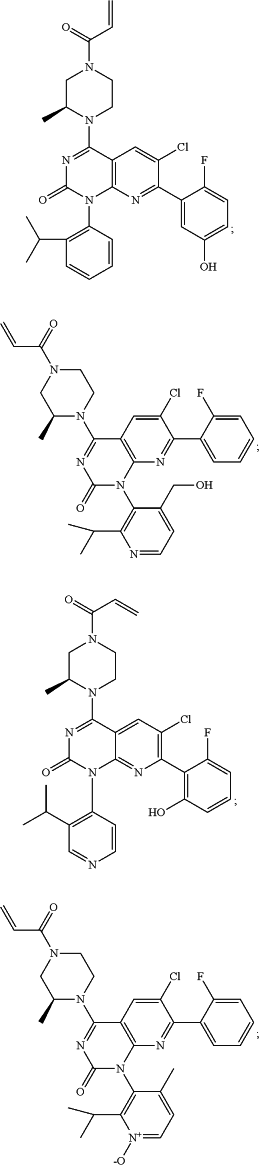                                                                        or a stereoisomer thereof, an atropisomer thereof, a pharmaceutically acceptable salt thereof, a pharmaceutically acceptable salt of the stereoisomer thereof, or a pharmaceutically acceptable salt of the atropisomer thereof; or
    or a stereoisomer thereof, an atropisomer thereof, a pharmaceutically acceptable salt thereof, a pharmaceutically acceptable salt of the stereoisomer thereof, or a pharmaceutically acceptable salt of the atropisomer thereof; or
 or a stereoisomer thereof, an atropisomer thereof, a pharmaceutically acceptable salt thereof, a pharmaceutically acceptable salt of the stereoisomer thereof, or a pharmaceutically acceptable salt of the atropisomer thereof; or
 or a stereoisomer thereof, an atropisomer thereof, a pharmaceutically acceptable salt thereof, a pharmaceutically acceptable salt of the stereoisomer thereof, a pharmaceutically acceptable salt of the atropisomer thereof; or
  or a stereoisomer thereof, an atropisomer thereof, a pharmaceutically acceptable salt thereof, a pharmaceutically acceptable salt of the stereoisomer thereof, a pharmaceutically acceptable salt of the atropisomer thereof; or
     or a stereoisomer thereof, an atropisomer thereof, a pharmaceutically acceptable salt thereof, a pharmaceutically acceptable salt of the stereoisomer thereof, or a pharmaceutically acceptable salt of the atropisomer thereof; or
 or a stereoisomer thereof, an atropisomer thereof, a pharmaceutically acceptable salt thereof, a pharmaceutically acceptable salt of the stereoisomer thereof, or a pharmaceutically acceptable salt of the atropisomer thereof; or
 or a stereoisomer thereof, an atropisomer thereof, a pharmaceutically acceptable salt thereof, a pharmaceutically acceptable salt of the stereoisomer thereof, or a pharmaceutically acceptable salt of the atropisomer thereof; or
   or a stereoisomer thereof, an atropisomer thereof, a pharmaceutically acceptable salt thereof, a pharmaceutically acceptable salt of the stereoisomer thereof, or a pharmaceutically acceptable salt of the atropisomer thereof, or
 or a stereoisomer thereof, an atropisomer thereof, a pharmaceutically acceptable salt thereof, a pharmaceutically acceptable salt of the stereoisomer thereof, or a pharmaceutically acceptable salt of the atropisomer thereof, or
 or a stereoisomer thereof, an atropisomer thereof, a pharmaceutically acceptable salt thereof, a pharmaceutically acceptable salt of the stereoisomer thereof, or a pharmaceutically acceptable salt of the atropisomer thereof;
wherein the cancer is mediated by a KRAS G12C mutation; and
wherein the cancer is non-small cell lung cancer, small intestine cancer, appendix cancer, colorectal cancer, endometrial cancer, pancreatic cancer, skin cancer, gastric cancer, nasal cavity cancer, bile duct cancer, or brain cancer.
|