| CPC H01M 4/58 (2013.01) [C01C 3/12 (2013.01); C01P 2004/03 (2013.01); C01P 2006/40 (2013.01); H01M 2004/028 (2013.01)] | 9 Claims |
|
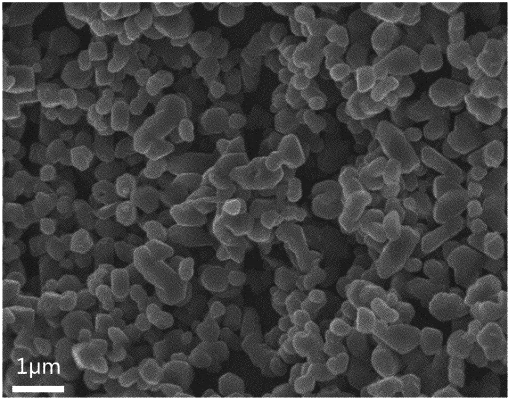
1. A preparation method of a positive electrode material for Prussian blue analogue sodium ion battery, comprising steps of:
adding a first nonionic surfactant and an antioxidant to a sodium ferrocyanide solution to obtain a first solution;
adding a second nonionic surfactant to a transition metal salt solution to obtain a second solution;
under a protective gas atmosphere, adding the second solution to the first solution for precipitation reaction, aging after the reaction is finished, collecting a precipitate, and washing; and
vacuum drying the washed precipitate, then soaking in an alcohol solution containing sodium alkoxide, filtering, and evaporating to dryness to obtain a positive electrode material for Prussian blue analogue sodium ion battery;
wherein the first nonionic surfactant and the second nonionic surfactant are independently one or both of polyethylene glycol and polyoxyethylene alkyl amide alcohol; the first nonionic surfactant is the same as the second nonionic surfactant; molecular weights of the first nonionic surfactant and the second nonionic surfactant are both ≥1500 g/mol; a concentration of the first nonionic surfactant in the first solution and a concentration of the second nonionic surfactant in the second solution are both 0.001-0.1 mol/L.
|