| CPC H01M 10/54 (2013.01) [C01F 11/22 (2013.01); C22B 1/02 (2013.01); C22B 1/248 (2013.01); C22B 3/22 (2013.01); C22B 7/001 (2013.01); C22B 7/007 (2013.01); C22B 7/008 (2013.01)] | 8 Claims |
|
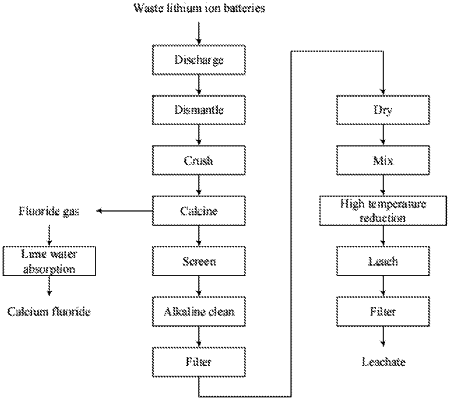
1. A method for recovering metals from waste lithium ion batteries, including the following steps:
step 1, pre-treatment, comprising:
short-circuit discharging, dismantling, crushing, roasting, and screening on waste lithium ion batteries to obtain active electrode powders; wherein the waste lithium ion battery comprises copper and aluminum, and the waste lithium ion battery is one or more of waste lithium nickel oxide lithium ion battery, lithium cobalt oxide lithium ion battery, lithium manganese oxide lithium ion battery, and lithium nickel cobalt manganese oxide lithium ion battery;
step 2, alkaline cleaning and filtering, comprising:
using alkaline solution to clean the active electrode powders, then filtering to remove copper and aluminum from the active electrode powders;
step 3, drying and mixing, comprising:
drying the active electrode powder after step 2, mixing the dried active electrode powder with starch and concentrated sulfuric acid in a predetermined proportion and stirring to obtain a mixed material,
wherein the predetermined proportion comprises:
the dried active electrode powders are mixed with starch, wherein an amount of starch added into the dried active electrode powders is controlled at 5-20 wt. %, and then concentrated sulfuric acid is added at a first solid-liquid ratio of 50-300 g/L and stirred;
step 4, high temperature reduction, comprising:
putting the mixed material into a corundum crucible, then moving the corundum crucible into a tube furnace to calcine, and controlling the atmosphere of the tube furnace during calcination,
wherein controlling the atmosphere of the tube furnace comprises controlling the calcination temperature at 300-800° C., heating rate at 10° C./min, and calcination time at 30-180 min; wherein the atmosphere is a mixture of O2 and N2, and the volume fraction of O2 is greater than 20 and not more than 50; and
step 5, water leaching and filtration, comprising:
taking out a product obtained from calcination in the step 4 and using deionized water to extract leachate and leaching residue with valence metal ions, and then obtaining the leachate after filtering.
|