| CPC G16H 10/40 (2018.01) [G01N 33/58 (2013.01); G06F 16/2379 (2019.01); G06Q 50/04 (2013.01); G16H 20/10 (2018.01); G06Q 2220/00 (2013.01)] | 20 Claims |
|
1. A computer assisted method of authenticating a therapeutic agent at a point of care, thereby validating therapy administration at the point of care, the computer assisted method comprising:
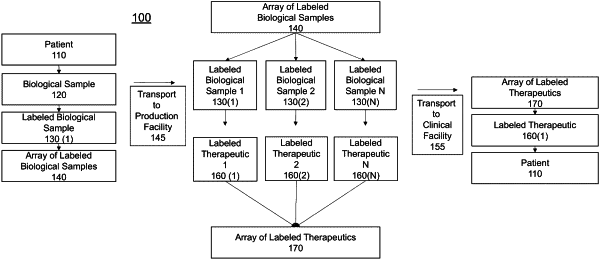
obtaining the biological sample from a patient;
labeling the biological sample with one or more first additives by inserting the one or more first additives into a container comprising the biological sample, wherein the one or more first additives uniquely label the biological sample among a population of biological samples, and wherein the one or more first additives include at least one of a primer, a monoclonal antibody, a rare earth metal, an isotope, or an autologous mitochondria;
obtaining, via at least one processor, first identifying digital data for the biological sample, wherein the identifying digital data is subjected to a sample tracking protocol to produce a sample tracking chain data structure stored in at least one computer memory, wherein the sample tracking chain data structure is indexed and thereby searchable for the first identifying digital data and the one or more first additives;
generating a current state block of the distributed digital ledger by executing a secure hash function on at least the one or more first additives and intrinsic biological data of the biological sample;
providing the labeled biological sample to a manufacturing facility for generation of a labeled patient-specific therapeutic, wherein the labeled therapeutic is subjected to the sample tracking protocol and thereby added, via the at least one processor, to the sample tracking chain data structure;
receiving the labeled therapeutic from the manufacturing facility, wherein the labeled therapeutic is associated with second identifying digital data;
performing an assay on the labeled therapeutic at the point of care, thereby validating the labeled therapeutic for administration to the patient, by detecting the presence of one or more second additives via the assay which uniquely label the therapeutic among a population of therapeutics, wherein the validation is subjected to the sample tracking protocol and thereby added, via the at least one processor, to the sample tracking chain data structure, the validation includes validating a timestamp of a state block of the distributed digital ledger associated with the second identifying digital data, based on an external public ledger, to confirm the state block associated with the second identifying digital data has not been modified or tampered with, and the sample tracking chain data structure is indexed and thereby searchable for the second identifying digital data and the one or more second additives.
|