| CPC G01N 33/58 (2013.01) [C07K 16/2812 (2013.01); C07K 16/2815 (2013.01); C08G 61/02 (2013.01); C09B 57/00 (2013.01); C09B 68/41 (2013.01); C09B 69/00 (2013.01); C12Q 1/6818 (2013.01); C12Q 1/682 (2013.01); G01N 33/582 (2013.01); H10K 85/151 (2023.02); C08G 2261/1424 (2013.01); C08G 2261/3142 (2013.01); C08G 2261/411 (2013.01); H10K 85/115 (2023.02)] | 25 Claims |
|
13. A conjugated polymer having the structure of the formula:
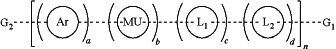 wherein:
Ar is polycyclic repeat unit comprising an ethylene glycol oligomer side group;
MU is a polymer modifying repeat unit or band gap modifying repeat unit that is evenly or randomly distributed along the polymer main chain and is optionally substituted with one or more optionally substituted substituents selected from halogen, hydroxyl, C1-C12 alkyl, C2-C12 alkene, C2-C12 alkyne, C3-C12 cycloalkyl, C1-C12 haloalkyl, C1-C12 alkoxy, C2-C18 (hetero) aryloxy, C2-C18 (hetero)arylamino, a C2-C18 (hetero) aryl group and (CH2)x′(OCH2CH2)y′OCH3 where x′ is independently an integer from 0-20 and y′ is independently an integer from 0 to 50;
optional linkers L1 and L2 are each independently an aryl or a heteroaryl group evenly or randomly distributed along the polymer main chain;
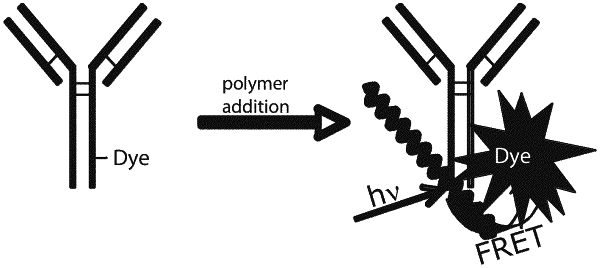
G1 and G2 are each independently selected from hydrogen, halogen, alkyne, optionally substituted aryl, optionally substituted heteroaryl, halogen substituted aryl, boronic acid substituted aryl, boronic ester substituted aryl, boronic ester, boronic acid, optionally substituted fluorene and aryl or heteroaryl substituted with one or more pendant chains, wherein at least one of G1 and G2 comprises a conjugated biomolecule, wherein at least one of G1 and G2 is distinct from any repeat unit;
n is an integer so that the conjugated polymer has a molecular weight ranging from 5,000 g/mol to 100,000 g/mol;
a, b, c and d define the mol % of each unit within the structure which each can be evenly or randomly repeated and where a is a mol % from 10 to 100%, b is a mol % from 0 to 90%, and each c and d are mol % from 0 to 25%; and
the conjugated polymer has a water solubility in excess of 10 mg/ml.
|