| CPC C12N 9/96 (2013.01) [C07C 231/12 (2013.01); C07C 245/18 (2013.01); C07C 269/06 (2013.01); C07C 319/12 (2013.01); C07D 207/46 (2013.01); C07K 1/13 (2013.01)] | 24 Claims |
|
1. A compound of formula I:
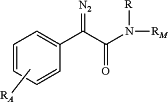 or salts thereof,
where:
R is hydrogen, an alkyl group, alkenyl group, or an alkynyl group;
RM is M or -L-M, where M is a polymer or a non-polymeric organic group, having from 1 to 100 carbon atoms and optionally nitrogen, oxygen or sulfur atoms, and -L is a divalent linker moiety having from 1-30 carbon atoms and optionally nitrogen, oxygen or sulfur atoms; and
RA represents 1 to 3 non-hydrogen substituents on the phenyl ring, wherein the non-hydrogen substituents are selected from the group consisting of alkyl, cycloalkyl, alkoxy, cycloalkoxy, aryl, arylalkyl, haloalkyl, haloalkoxy, heterocyclyl and RP—CO—NH—, where the alkyl, cycloalkyl, alkoxy, cycloalkoxy, aryl, arylalkyl and heterocyclyl groups are optionally substituted;
wherein optional substitution is substitution with one or more groups selected from oxo, thiox, -sulfhydryl, hydroxy, alkyl, alkoxy, alkenyl, alkenyloxy, alkynyl, alkynyloxy, aryl, aryloxy, heteroaryl, heteroaryloxy, carbocyclyl, carbocyclyloxy, heterocyclyl, heterocyclyloxy, alkylthio, alkenylthio, alkynylthio, arylthio, thioheteroaryl, thiocarbocyclyl, thioheterocyclyl, —CORs, —COH, —OCORs, —OCOH, —CO—ORs, —CO—OH, —CO—O—CO-Rs, —CON(Rs)2, —CONHRs, —CONH2, —NRs-CORs, —NHCORs, —NHRs, —N(Rs)2, —O—SO2-Rs, —SO2-Rs, —SO2—NHRs, —SO2—N(Rs)2, —NRs-SO2-Rs, —NH—SO2-Rs, —NRsCO—N(Rs)2, —NH—CO—NHRs, —O—PO(ORs)2, —O—PO(ORs)(N(Rs)2), —O—PO(N(Rs)2)2, —N—PO(ORs)2, —N—PO(ORs)(N(Rs)2), —P(Rs)2, —B(OH)2, —B(OH)(ORs), and —B(ORs)2, where each Rs independently is an alkyl, alkenyl, alkynyl, aryl, heteroaryl, carbocyclyl, or heterocyclyl group or two Rs within the same substituent can together form a carbocyclic or heterocyclic ring having 3 to 10 ring atoms.
|