| CPC C07D 487/04 (2013.01) | 18 Claims |
|
1. A compound according to general formula (I),
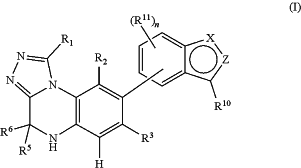 wherein
R1 represents H; C1-10-alkyl; C3-10-cycloalkyl; 3 to 7 membered heterocycloalkyl; aryl; or 5 or 6-membered heteroaryl;
wherein C3-10-cycloalkyl, 3 to 7 membered heterocycloalkyl, aryl and 5 or 6-membered heteroaryl can optionally be bridged via C1-6-alkylene;
R2 represents H; F; Cl; Br; I; CN; C1-10-alkyl; C3-10-cycloalkyl; O—C1-10-alkyl; N(H)(C1-10-alkyl), N(C1-10-alkyl)2; C(O)—C1-10-alkyl; C(O)—O—C1-10-alkyl; C(O)—NH2; C(O)—N(H)(C1-10-alkyl); C(O)—N(C1-10-alkyl)2; O—C3-10-cycloalkyl; N(H)(C3-10-cycloalkyl), N(C1-10-alkyl)(C3-10-cycloalkyl); C(O)—C3-10-cycloalkyl; C(O)—O—C3-10-cycloalkyl; C(O)—N(H)(C3-10-cycloalkyl) or C(O)—N(C1-10-alkyl)(C3-10-cycloalkyl);
wherein C3-10-cycloalkyl can optionally be bridged via C1-6-alkylene;
R3 represents H; F; Cl; Br; I; CN; C1-10-alkyl; C3-10-cycloalkyl; O—C1-10-alkyl; N(H)(C1-10-alkyl); N(C1-10-alkyl)2; C(O)—C1-10-alkyl; C(O)—O—C1-10-alkyl; C(O)—NH2; C(O)—N(H)(C1-10-alkyl); C(O)—N(C1-10-alkyl)2; O—C3-10-cycloalkyl; N(H)(C3-10-cycloalkyl), N(C1-10-alkyl)(C3-10-cycloalkyl); C(O)—C3-10-cycloalkyl; C(O)—O—C3-10-cycloalkyl; C(O)—N(H)(C3-10-cycloalkyl) or C(O)—N(C1-10-alkyl)(C3-10-cycloalkyl);
wherein C3-10-cycloalkyl can optionally be bridged via C1-6-alkylene;
R5 and R6 represent independently from one another H or unsubstituted C1-4-alkyl;
X represents N or NR7;
Z represents N, NR7 or CR9;
with the proviso that
when X represents NR7, Z represents N or CR9;
when X represents N, Z represents NR7;
R7 represents H or L-R8; wherein
L represents bond; S(O); S(O)2; C1-6-alkylene; C(O); C1-6-alkylene-C(O); C(O)—O; C1-6-alkylene-C(O)—O; C1-6-alkylene-N(H)—C(O); C1-6-alkylene-N(C1-10-alkyl)-C(O); C1-6-alkylene-N(H)—C(O)—O; C1-6-alkylene-N(C1-10-alkyl)-C(O)—O; O; NH or N(C1-10-alkyl);
R8 represents C1-10-alkyl; C3-10-cycloalkyl or 3 to 7 membered heterocycloalkyl;
wherein C3-10-cycloalkyl and 3 to 7 membered heterocycloalkyl can optionally be bridged via C1-6-alkylene;
R9 and R10 represent independently from one another H; F; Cl; Br; I; CN; C1-10-alkyl; C3-10-cycloalkyl; 3 to 7 membered heterocycloalkyl; S(O)-(C1-10-alkyl); S(O)-(C3-10-cycloalkyl); S(O)-(3 to 7-membered heterocycloalkyl); S(O)2—(C1-10-alkyl); S(O)2—(C3-10-cycloalkyl); S(O)2-(3 to 7-membered heterocycloalkyl); P(O)-(C1-10-alkyl)2; P(O)(C1-10-alkyl)(C3-10-cycloalkyl); P(O)(C1-10-alkyl)(3 to 7-membered heterocycloalkyl); P(O)—(O—C1-10-alkyl)2; P(O)(O—C1-10-alkyl)(O—C3-10-cycloalkyl); P(O)(O—C1-10-alkyl)(O-(3 to 7-membered heterocycloalkyl)); O—C1-10-alkyl; S—C1-10-alkyl; N(H)(C1-10-alkyl), N(C1-10-alkyl)2; C(O)—C1-10-alkyl; C(O)—O—C1-10-alkyl; C(O)—NH2; C(O)—N(H)(C1-10-alkyl); C(O)—N(C1-10-alkyl)2; O—C3-10-cycloalkyl; N(H)(C3-10-cycloalkyl), N(C1-10-alkyl)(C3-10-cycloalkyl); C(O)—C3-10-cycloalkyl;
C(O)—O—C3-10-cycloalkyl; C(O)—N(H)(C3-10-cycloalkyl); C(O)—N(C1-10-alkyl)(C3-10-cycloalkyl); O-3 to 7-membered heterocycloalkyl; N(H)(3 to 7-membered heterocycloalkyl), N(C1-10-alkyl)(3 to 7-membered heterocycloalkyl); C(O)-3 to 7-membered heterocycloalkyl; C(O)—O-(3 to 7-membered heterocycloalkyl); C(O)—N(H)(3 to 7-membered heterocycloalkyl) or C(O)—N(C1-10-alkyl)(3 to 7-membered heterocycloalkyl);
wherein C3-10-cycloalkyl and 3 to 7 membered heterocycloalkyl can optionally be bridged via C1-6-alkylene;
R11 represents F; Cl; Br; I; CN; C1-10-alkyl; O—C1-10-alkyl; NO2; OH, NH2; C3-10-cycloalkyl; 3 to 7-membered heterocycloalkyl; S(O)-(C1-10-alkyl); S(O)-(C3-10-cycloalkyl); S(O)-(3 to 7-membered heterocycloalkyl); S(O)2—(C1-10-alkyl); S(O)2—(C3-10-cycloalkyl); S(O)2-(3 to 7-membered heterocycloalkyl); P(O)-(C1-10-alkyl)2; P(O)(C1-10-alkyl)(C3-10-cycloalkyl); P(O)(C1-10-alkyl)(3 to 7-membered heterocycloalkyl); P(O)—(O—C1-10-alkyl)2; P(O)(O—C1-10-alkyl)(O—C3-10-cycloalkyl); P(O)(O—C1-10-alkyl)(O-(3 to 7-membered heterocycloalkyl)); O—C1-10-alkyl; N(H)(C1-10-alkyl), N(C1-10-alkyl)2; C(O)—C1-10-alkyl; C(O)—O—C1-10-alkyl; C(O)—NH2; C(O)—N(H)(C1-10-alkyl); C(O)—N(C1-10-alkyl)2; O—C3-10-cycloalkyl; N(H)(C3-10-cycloalkyl), N(C1-10-alkyl)(C3-10-cycloalkyl); C(O)—C3-10-cycloalkyl; C(O)—O—C3-10-cycloalkyl; C(O)—N(H)(C3-10-cycloalkyl); C(O)—N(C1-10-alkyl)(C3-10-cycloalkyl); O-3 to 7-membered heterocycloalkyl; N(H)(3 to 7-membered heterocycloalkyl), N(C1-10-alkyl)(3 to 7-membered heterocycloalkyl); C(O)-3 to 7-membered heterocycloalkyl; C(O)—O-(3 to 7-membered heterocycloalkyl); C(O)—N(H)(3 to 7-membered heterocycloalkyl) or C(O)—N(C1-10-alkyl)(3 to 7-membered heterocycloalkyl);
wherein C3-10-cycloalkyl and 3 to 7 membered heterocycloalkyl can optionally be bridged via C1-6-alkylene;
n represents 0, 1, 2 or 3;
wherein C1-10-alkyl, C1-4-alkyl and C1-6-alkylene in each case independently from one another is linear or branched, saturated or unsaturated;
wherein C1-10-alkyl, C1-4-alkyl, C1-6-alkylene, C3-10-cycloalkyl and 3 to 7 membered heterocycloalkyl in each case independently from one another are unsubstituted or mono- or polysubstituted with one or more substituents selected from F; Cl; Br; I; CN; C1-6-alkyl; CF3; CF2H; CFH2; CF2Cl; CFCl2; C(O)—C1-6-alkyl; C(O)—OH; C(O)—OC1-6-alkyl; C(O)—NH2; C(O)—N(H)(C1-6-alkyl); C(O)—N(C1-6-alkyl)2; OH; ═O; OCF3; OCF2H; OCFH2; OCF2Cl; OCFCl2; O—C1-6-alkyl; O—C(O)—C1-6-alkyl; O—C(O)—O—C1-6-alkyl; O—(CO)—N(H)(C1-6-alkyl); O—C(O)—N(C1-6-alkyl)2; O—S(O)2—NH2; O—S(O)2—N(H)(C1-6-alkyl); O—S(O)2—N(C1-6-alkyl)2; NH2; N(H)(C1-6-alkyl); N(C1-6-alkyl)2; N(H)—C(O)—C1-6-alkyl; N(H)—C(O)—O—C1-6-alkyl; N(H)—C(O)—NH2; N(H)—C(O)—N(H)(C1-6-alkyl); N(H)—C(O)—N(C1-6-alkyl)2; N(C1-6-alkyl)-C(O)—C1-6-alkyl; N(C1-6-alkyl)-C(O)—O—C1-6-alkyl; N(C1-6-alkyl)-C(O)—NH2; N(C1-6-alkyl)-C(O)—N(H)(C1-6-alkyl); N(C1-6-alkyl)-C(O)—N(C1-6-alkyl)2; N(H)—S(O)2OH; N(H)—S(O)2—C1-6-alkyl; N(H)—S(O)2—O—C1-6-alkyl; N(H)—S(O)2—NH2; N(H)—S(O)2—N(H)(C1-6-alkyl); N(H)—S(O)2N(C1-6-alkyl)2; N(C1-6-alkyl)-S(O)2—OH; N(C1-6-alkyl)-S(O)2—C1-6-alkyl; N(C1-6-alkyl)-S(O)2—O—C1-6-alkyl; N(C1-6-alkyl)-S(O)2—NH2; N(C1-6-alkyl)-S(O)2—N(H)(C1-6-alkyl); N(C1-6-alkyl)-S(O)2—N(C1-6-alkyl)2; SCF3; SCF2H; SCFH2; S—C1-6-alkyl; S(O)—C1-6-alkyl; S(O)2—C1-6-alkyl; S(O)2—OH; S(O)2—O—C1-6-alkyl; S(O)2—NH2; S(O)2—N(H)(C1-6-alkyl); S(O)2—N(C1-6-alkyl)2; C3-6-cycloalkyl; 3 to 6-membered heterocycloalkyl; phenyl; 5 or 6-membered heteroaryl; O—C3-6-cycloalkyl; O-(3 to 6-membered heterocycloalkyl); O-phenyl; O-(5 or 6-membered heteroaryl); C(O)—C3-6-cycloalkyl; C(O)-(3 to 6-membered heterocycloalkyl); C(O)-phenyl; C(O)-(5 or 6-membered heteroaryl); S(O)2—(C3-6-cycloalkyl); S(O)2-(3 to 6-membered heterocycloalkyl); S(O)2-phenyl or S(O)2-(5 or 6-membered heteroaryl);
wherein aryl and 5 or 6-membered heteroaryl in each case independently from one another are unsubstituted or mono- or polysubstituted with one or more substituents selected from F; Cl; Br; I; CN; C1-6-alkyl; CF3; CF2H; CFH2; CF2Cl; CFCl2; C1-4-alkylene-CF3; C1-4-alkylene-CF2H; C1-4-alkylene-CFH2; C(O)—C1-6-alkyl; C(O)—OH; C(O)—OC1-6-alkyl; C(O)—N(H)(OH); C(O)—NH2; C(O)—N(H)(C1-6-alkyl); C(O)—N(C1-6-alkyl)2; OH; OCF3; OCF2H; OCFH2; OCF2Cl; OCFCl2; O—C1-6-alkyl; O—C3-6-cycloalkyl; O-(3 to 6-membered heterocycloalkyl); NH2; N(H)(C1-6-alkyl); N(C1-6-alkyl)2; N(H)—C(O)—C1-6-alkyl; N(C1-6-alkyl)-C(O)—C1-6-alkyl; N(H)—C(O)—NH2; N(H)—C(O)—N(H)(C1-6-alkyl); N(H)—C(O)—N(C1-6-alkyl)2; N(C1-6-alkyl)-C(O)—N(H)(C1-6-alkyl); N(C1-6-alkyl)-C(O)—N(C1-6-alkyl)2; N(H)—S(O)2—C1-6-alkyl; SCF3; S—C1-6-alkyl; S(O)—C1-6-alkyl; S(O)2—C1-6-alkyl; S(O)2—NH2; S(O)2—N(H)(C1-6-alkyl); S(O)2—N(C1-6-alkyl)2; C3-6-cycloalkyl; C1-4-alkylene-C3-6-cycloalkyl; 3 to 6-membered heterocycloalkyl; C1-4-alkylene-(3 to 6-membered heterocycloalkyl); phenyl or 5 or 6-membered heteroaryl;
in the form of the free compound or a physiologically acceptable salt thereof.
|