| CPC C07D 471/04 (2013.01) [C07D 239/545 (2013.01); C07D 401/12 (2013.01); C07D 401/14 (2013.01); C07D 403/12 (2013.01); C07D 405/14 (2013.01); C07D 491/048 (2013.01); C07D 495/04 (2013.01); C07D 519/00 (2013.01)] | 17 Claims |
|
1. A compound having the following Structure (I):
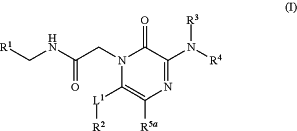 or a stereoisomer, tautomer, or pharmaceutically acceptable salt thereof, wherein:
R1 is a substituted or unsubstituted heteroaryl,
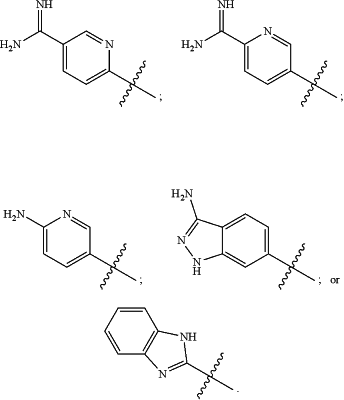
wherein the substituted or unsubstituted heteroaryl is selected from the group consisting of:
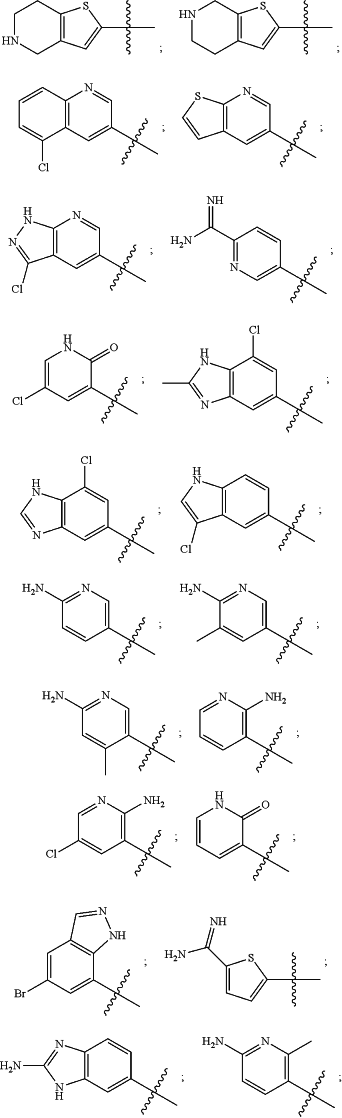 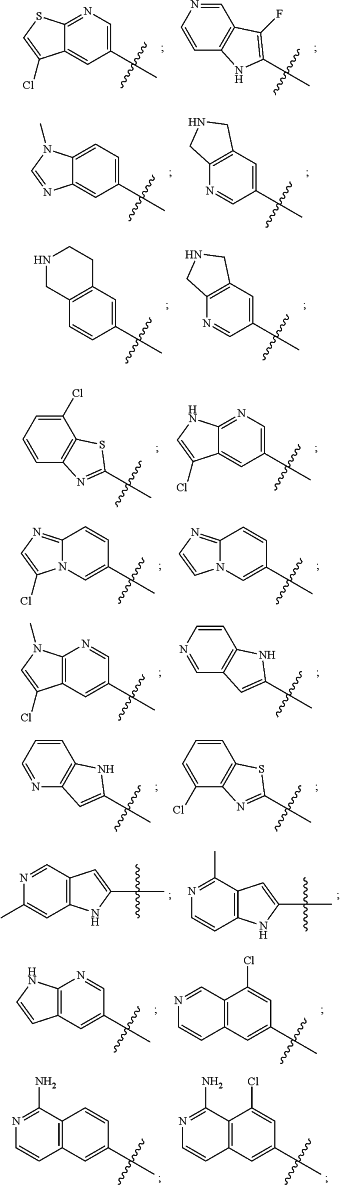 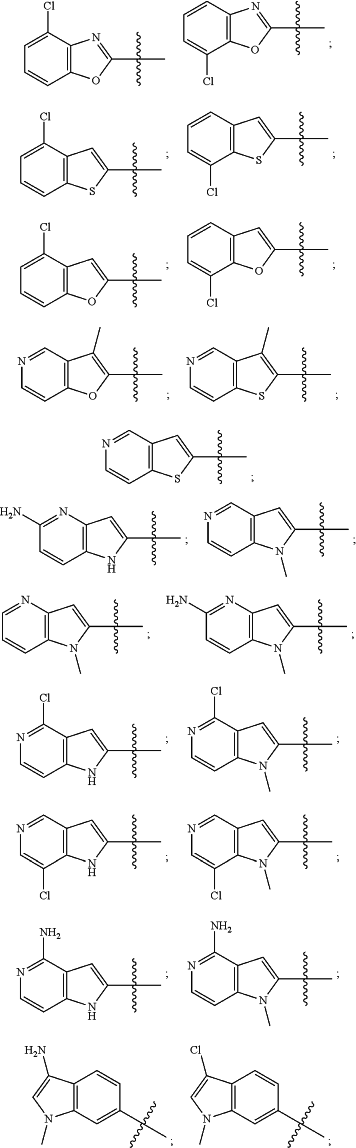 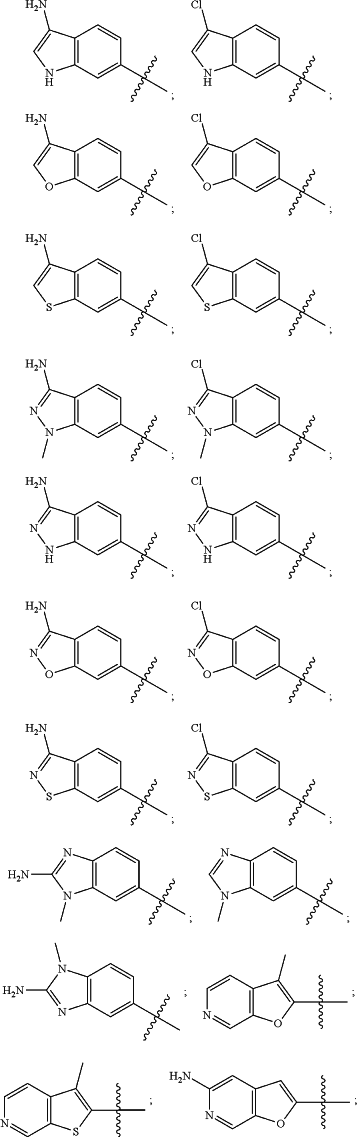 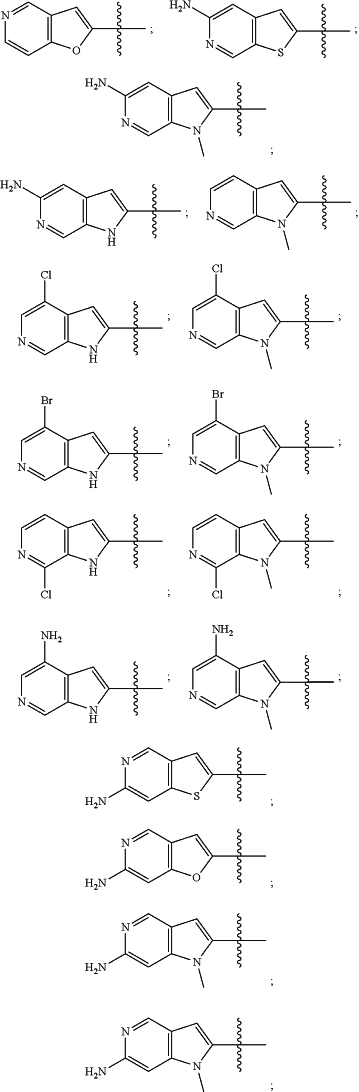 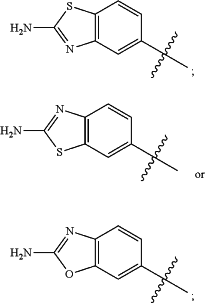 R2 is a substituted or unsubstituted aryl, or a substituted or unsubstituted heteroaryl,
wherein the substituted aryl of R2 is substituted with one or more of R2a, R2b, R2c, R2d, or R2e wherein R2a, R2b, R2c, R2d, and R2e are each independently selected from the group consisting of C1-6 alkyl, C1-6 deuterated alkyl, C2-6 alkenyl, C2-6 alkynyl, halo, C1-6 haloalkyl, aminylalkyl, hydroxyalkyl, phosphate, phosphonalkyl, phosphoalkyl, cyano, nitro, ORa, SRa, C(O)Ra, C(O)NRaRb, C(O)ORa, OC(O)Ra, OC(O)ORa, OC(O)NRaRb, NRaRb, N(Ra)C(O)Rb, N(Ra)C(O)NRbRc, N(Ra)C(O)ORb, C(═NRa)NRbRe, C(═NORa)NRbRe, C(═NOC(O)Ra)NRbRc, C(═NRa)N(Rb)C(O)ORa, N(Ra)C(═NRb)NRaRd, S(O)Ra, S(O)NRaRb, S(O)2Ra, N(Ra)S(O)2Rb, S(O)2NRaRb, oxo, substituted or unsubstituted C6-10 aryl, substituted or unsubstituted C6-10 arylalkyl, substituted or unsubstituted C6-10 aryloxy, substituted or unsubstituted C6-10 arylalkoxy, substituted or unsubstituted 5-10 membered heteroaryl, substituted or unsubstituted C3-10 cycloalkyl, and substituted or unsubstituted 4-10 membered heterocyclyl,
wherein Ra, Rb, Re, and Rd, are, at each occurrence, independently selected from the group consisting of hydrogen, C1-6 alkyl, C2-6 alkenyl, C2-6 alkynyl, hydroxyl, C1-6 alkoxy, aryl, arylalkyl, C1-6 haloalkyl, C1-6 haloalkoxy, C1-6 hydroxyalkyl, cycloalkyl, heterocyclyl, and heteroaryl, and
wherein R2a, R2b, R2c, R2d, or R2e is optionally substituted with one or more substituents selected from the group consisting of halo, CN, ORe, SRe, C(O)Re, C(O)NReRf, C(O)ORe, OC(O)Re, OC(O)NReRf, NReRf, NReC(O)Rf, NReC(O)NRfRg, NReC(O)ORf, C(═NRe)NRfRg, NReC(═NRf)NRgRh, S(O)Re, S(O)NReRf, S(O)2Re, NReS(O)2Rf, S(O)2NReRf and oxo when R2aR2b, R2c, R2d, or R2e is a substituted C6-10 aryl, a substituted C6-10 arylalkyl, a substituted C6-10 aryloxy, a substituted C6-10 arylalkoxy, a substituted 5-10 membered heteroaryl, a substituted C3-10 cycloalkyl, and a substituted 4-10 membered heterocyclyl,
wherein Re, Rf, Rg, and Rh are, at each occurrence, independently selected from the group consisting of hydrogen, C1-6 alkyl, C2-6 alkenyl, C2-6 alkynyl, hydroxyl, C1-6 alkoxy, aryl, arylalkyl, C1-6 haloalkyl, C1-6 haloalkoxy, C1-6 hydroxyalkyl, cycloalkyl, heterocyclyl, and heteroaryl,
wherein the substituted heteroaryl of R2 is substituted with one or more of R2a, R2b, R2c, R2d, or R2e wherein R2a, R2b, R2c, R2d, and R2e are each independently selected from the group consisting of C1-6 alkyl, C1-6 deuterated alkyl, C2-6 alkenyl, C2-6 alkynyl, halo, C1-6 haloalkyl, aminylalkyl, hydroxyalkyl, cyano, nitro, ORa, SRa, C(O)Ra, C(O)NRaRb, C(O)ORa, OC(O)Ra, OC(O)ORa, OC(O)NRaRb, NRaRb, N(Ra)C(O)Rb, N(Ra)C(O)NRbRc, N(Ra)C(O)ORb, C(═NRa)NRbRc, C(═NORa)NRbRc, C(═NOC(O)Ra)NRbRe, C(═NRa)N(Rb)C(O)ORc, N(Ra)C(═NRb)NRaRd, S(O)Ra, S(O)NRaRb, S(O)2Ra, N(Ra)S(O)2Rb, S(O)2NRaRb, oxo, substituted or unsubstituted C6-10 aryl, substituted or unsubstituted C6-10 arylalkyl, substituted or unsubstituted C6-10 aryloxy, substituted or unsubstituted C6-10 arylalkoxy, substituted or unsubstituted 5-10 membered heteroaryl, substituted or unsubstituted C3-10 cycloalkyl, and substituted or unsubstituted 4-10 membered heterocyclyl,
wherein Ra, Rb, Rc, and Rd, are, at each occurrence, independently selected from the group consisting of hydrogen, C1-6 alkyl, C2-6 alkenyl, C2-6 alkynyl, hydroxyl, C1-6 alkoxy, aryl, arylalkyl, C1-6 haloalkyl, C1-6 haloalkoxy, C1-6 hydroxyalkyl, cycloalkyl, heterocyclyl, and heteroaryl, and
wherein R2a, R2b, R2c, R2d, or R2e is optionally substituted with one or more substituents selected from the group consisting of halo, CN, ORe, SRe, C(O)Re, C(O)NReRf, C(O)ORe, OC(O)Re, OC(O)NReRf, NReRf, NReC(O)Rf, NReC(O)NRfR8, NReC(O)ORf, C(═NRe)NRfRg, NReC(═NRf)NRgRh, S(O)Re, S(O)NReRf, S(O)2Re, NReS(O)2Rf, S(O)2NReRf and oxo when R2aR2b, R2c, R2d, or R2e is a substituted C6-10 aryl, a substituted C6-10 arylalkyl, a substituted C6-10 aryloxy, a substituted C6-10 arylalkoxy, a substituted 5-10 membered heteroaryl, a substituted C3-10 cycloalkyl, and a substituted 4-10 membered heterocyclyl,
wherein Re, Rf, Rg, and Rh are, at each occurrence, independently selected from the group consisting of hydrogen, C1-6 alkyl, C2-6 alkenyl, C2-6 alkynyl, hydroxyl, C1-6 alkoxy, aryl, arylalkyl, C1-6 haloalkyl, C1-6 haloalkoxy, C1-6 hydroxyalkyl, cycloalkyl, heterocyclyl, and heteroaryl;
R3 is hydrogen or alkyl;
R4 is alkyl, an arylalkyl, a heterocyclyl substituted with substituents selected from the group consisting of a phenyl or a pyridinyl, or R3 and R4, together with the nitrogen to which they are attached, form a 4-10 membered heterocyclyl;
R5a is hydrogen or halo;
R5b is hydrogen, alkyl, haloalkyl, (C═O)alkyl, (C═O)Oalkyl, (C═O)cycloalkyl, (C═O)Ocycloalkyl, (C═O)aryl, (C═O)Oaryl, (C═O)heteroaryl, (C═O)Oheteroaryl, (C═O)heterocyclyl, (C═O)Oheterocyclyl, an aryl, a heteroaryl, a cycloalkyl, a heterocyclyl, an arylalkyl, a heteroarylalkyl, a cycloalkylalkyl, or a heterocyclylalkyl;
L1 is a direct bond, —CH2—, —S(O)t—, NR5b, —O—, —C═C—, or —C≡C—; and
t is 0, 1, or 2, and
wherein the aryl is a 6- to 18-membered monocyclic, bicyclic, tricyclic, or tetracyclic ring system comprising at least one aromatic ring, and which can comprise fused or bridged ring systems,
wherein the heteroaryl of R2, Ra, Rb, Rc, Rd, Re, Rf, Rg, Rh, and R5b, are a 5- to 14-membered monocyclic, bicyclic, tricyclic, or tetracyclic ring system consisting of at least one aromatic ring, one to thirteen carbon atoms, one to six heteroatoms selected from the group consisting of nitrogen, oxygen, and sulfur, and can comprise fused or bridged ring systems,
wherein the cycloalkyl is a non-aromatic 3- to 15-membered monocyclic or polycyclic ring system, which is saturated or unsaturated, and which can comprise fused or bridged ring systems, and
wherein the heterocyclyl is a 3- to 18-membered monocyclic, bicycylic, tricyclic, or tetracyclic ring system consisting of two to twelve carbon atoms and from one to six heteroatoms selected from the group consisting of nitrogen, oxygen, and sulfur, and which can comprise fused, bridged, and spiro ring systems,
provided that:
A) R2 does not have one of the following structures:
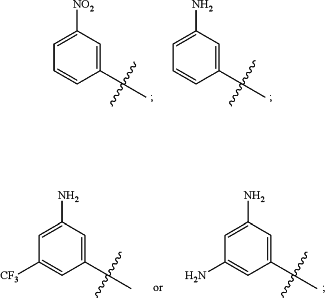 and
B) when R2 is unsubstituted phenyl, R1 does not have one of the following structures:
 |