| CPC C07D 401/06 (2013.01) [C07D 417/14 (2013.01)] | 19 Claims |
|
1. A method of treating a neurological disorder in a patient in need thereof comprising administering to the patient a therapeutically effective amount of a compound of Formula IIa:
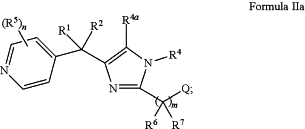 or a pharmaceutically acceptable salt thereof, wherein:
Q is —Cy, —C1-4 alkyl-Cy, —CF3, or —C1-4 alkyl-CF3;
R1 is H;
R2 is H or C1-4 alkyl;
R4 and R4a are each independently selected from H, halo, and C1-4 alkyl;
each R5 is independently selected from halo and C1-4 alkyl, wherein R5 is attached to a carbon atom;
R6 and R7 are each independently selected from H, —ORa, —NRcRd, C1-4 alkyl, and C1-4 haloalkyl;
Cy is selected from C6-10 aryl, C3-7 cycloalkyl, 5-6 membered heteroaryl, and 4-7 membered heterocycloalkyl, each optionally substituted by 1, 2, 3, 4, or 5 RCy substituents independently selected from halo, C1-4 alkyl, C1-4 haloalkyl, CN, NO2, ORa, SRa, C(O)Rb, C(O)NRcRd, C(O)ORa, OC(O)Rb, OC(O)NRcRd, NRcRd, NRcC(O)Rb, NRcC(O)ORa, NRcC(O)NRcRd, C(═NRe)Rb, C(═NRe)NRcRd, NRcC(═NRe)NRcRd, NRCS(O)Rb, NRCS(O)2Rb, NRCS(O)2NRcRd, S(O)Rb, S(O)NRcRd, S(O)2Rb, and S(O)2NRcRd,
or two adjacent Roy substituents together with the atoms to which they are attached form a fused phenyl, C3-7 cycloalkyl, 5-6 membered heteroaryl, or 4-7 membered heterocycloalkyl ring, each optionally substituted by 1, 2, 3, 4, or 5 substituents independently selected from halo, C1-4 alkyl, C1-4 haloalkyl, CN, NO2, ORa, SRa, C(O)Rb, C(O)NRcRd, C(O)ORa, OC(O)Rb, OC(O)NRcRd, NRcRd, NRcC(O)Rb, NRcC(O)ORa, NRcC(O)NRcRd, C(═NRe)Rb, C(═NRe)NRcRd, NRcC(═NRe)NRcRd, NRCS(O)Rb, NRCS(O)2Rb, NRCS(O)2NRcRd, S(O)Rb, S(O)NRcRd, S(O)2Rb, and S(O)2NRcRd,
each Ra, Rb, Rc, and Rd is independently selected from H, C1-4 alkyl, and C1-4 haloalkyl, wherein said C1-4 alkyl is optionally substituted with 1, 2, or 3 substituents independently selected from OH, CN, amino, halo, C1-4 alkyl, C1-4 alkoxy, C1-4 haloalkyl, and C1-4 haloalkoxy;
each Re is independently selected from H, C1-4 alkyl, and CN;
n is 0, 1, or 2; and
m is 1 or 2; wherein the neurological disorder is selected from amyotrophic lateral sclerosis (ALS), multiple sclerosis (MS), diabetic neuropathy, traumatic brain injury (TBI), ocular neuropathy, Parkinson's disease, Alzheimer's disease, and peripheral neuropathy.
|