| CPC C07D 231/56 (2013.01) [C07D 471/04 (2013.01); C07F 9/095 (2013.01)] | 28 Claims |
|
1. A compound of formula (I-AB):
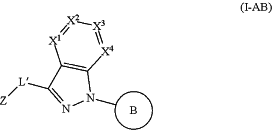 or a stereoisomer or tautomer thereof, or a pharmaceutically acceptable salt thereof, wherein:
L′ is *—N(R1)-L-**, wherein * denotes the point of attachment to Z, and ** denotes the point of attachment to
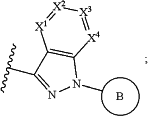 R1 is H or C1-6 alkyl;
X1 is N or CRs, wherein Rs is selected from H, deuterium, —CN, halo, C1-15alkyl, C1-6alkynyl, C6-20aryl, 5 to 15 membered heteroaryl, C3-20cycloalkyl, 3 to 15 membered heterocyclyl, —OH, —ORf, C1-15alkoxy, —NRdCORe, —CONRdRe, —SO2Rd, —SO2NRdRe, —NRdSO2Re, and —NRdRe;
wherein each of Rd, Re and Rf are independently H, C1-6alkyl, or C3-20cycloalkyl, wherein each of C1-6alkyl and C3-20cycloalkyl are independently optionally substituted with one or more halo, oxo, —OH, or —CN;
wherein the C1-15alkyl and C1-15alkoxy of Rs are each independently optionally substituted with one or more Rt1, wherein Rt1 is independently, at each occurrence, selected from halo, oxo, —OH, —OR1, —CN, —NRdRe, and 3 to 15 membered heterocyclyl optionally substituted with one or more —OH; wherein Rf1 is —C(O)CH2NRdRe, —C(O)C1-6alkyl, —P(O)(OH)2; wherein Rd and Re are each independently H or C1-6alkyl, and the C1-6alkyl of Rd or Re is optionally substituted with one or more halo, oxo, —OH, or —CN; and
wherein the C6-20aryl, 5 to 15 membered heteroaryl, C3-20cycloalkyl, and 3 to 15 membered heterocyclyl of Rs are each independently optionally substituted with one or more Rt2, wherein Rt2 is independently, at each occurrence, selected from halo, oxo, —OH, —CN, C(O)NH2, —C(O)NRdRe, —NRdRe, C1-6alkoxyl, 3 to 6 membered heterocyclyl, C3-6cycloalkyl, and C1-6alkyl; wherein the C1-6alkyl of Rt2 is optionally substituted with one or more —OH, C1-6alkoxyl, halo, oxo, —S(O)2CH3, or —NRdRe; wherein Rd and Re are each independently H, —C(O)CH3, —C(O)C1-6alkyl, or C1-6alkyl;
X2, X3, and X4 are each independently N, CH, or CD, provided that: 1) only one of X1, X2, X3, and X4 is N; or 2) X1 is N, X2 is CH, X3 is CH, and X4 is N; or 3) X1 is CRs and X2, X3, and X4 are each independently CH or CD;
B is phenyl or 5 to 6 membered heteroaryl, wherein the phenyl or 5 to 6 membered heteroaryl of B are each independently optionally substituted with one or more R, wherein R is independently at each occurrence selected from halo, C1-15alkyl, C1-6alkoxy, and S(Ry)5, wherein each Ry is halo, and wherein the C1-15alkyl or C1-6alkoxy of Rt is optionally substituted with one or more halo;
Z is —C(O)Rx, wherein Rx is C2-6alkenyl, optionally substituted with one or more substituents selected from C1-6alkyl, deuterium, —OH, C1-6alkoxyl, and halo; or Rx is C1-6alkyl, optionally substituted with one or more halo; or Rx is C1-6alkynyl optionally substituted with —OH; or Rx is cyclobutenyl, dihydrofuranyl, bicyclobutanyl, or cyclopentenyl; or
Z is S(O)2Rx1, wherein Rx1 is C2-6alkenyl; and
L is methylene, optionally substituted with one or more C1-6alkyl.
|