| CPC A61N 7/02 (2013.01) [A61N 7/00 (2013.01); A61N 2007/0056 (2013.01); A61N 2007/0078 (2013.01)] | 26 Claims |
|
1. A method of treating peripheral vascular disease by stimulating vasodilation within a patient, comprising:
providing a wearable non-invasive device comprising a flexible housing material and an array of ultrasound transducers operably attached to the flexible housing material;
positioning the device and the array of transducers proximate a skin surface of a patient above at least one target site angiosome below the knee where vasodilation is desired, and such that the flexible housing material and the array of ultrasound transducers conforms to the skin surface of one or more of the calf, ankle, and foot of the patient; and
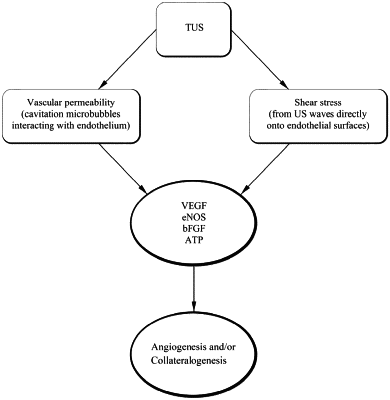
causing a therapeutically effective amount of therapeutic ultrasonic energy to be directed toward the target site angiosome without delivering a drug, to stimulate cavitation and shear stress within tissue at the target site angiosome, to stimulate the production and/or release of growth factors, angiogenic factors, tissue vascular endothelial growth factor (VEGF), endothelial nitric oxide synthase (eNOS), basic fibroblast growth factor (bFGF), or adenosine triphosphate (ATP) and to stimulate vasodilation within the patient, and
assessing acoustic or electrical parameters of individual transducers of the array of ultrasound transducers during therapy to determine the presence of bone or air in a near-field, and thereby reducing or ceasing power to one or more of the individual transducers if bone or air is found to be present in the near-field,
wherein said causing the therapeutically effective amount of therapeutic ultrasonic energy to be directed toward the target site angiosome occurs for at least 15 minutes per treatment session and for at least five treatment sessions,
wherein the therapeutic ultrasonic energy has a mechanical index in the range of 2 to 6,
wherein the therapeutic ultrasonic energy has a peak negative pressure between 1 MPa and 4 MPa,
wherein the therapeutic ultrasonic energy has a derated spatial-peak pulse-average intensity (Isppa) between 190 W/cm2 and 1,000 W/cm2, and
wherein each transducer of the array of ultrasound transducers is operated at a duty cycle of no more than 1%.
|