| CPC A61L 31/04 (2013.01) [A61L 15/26 (2013.01); A61L 15/44 (2013.01); A61L 15/64 (2013.01); A61L 27/18 (2013.01); A61L 27/54 (2013.01); A61L 27/58 (2013.01); A61L 27/60 (2013.01); B29C 48/18 (2019.02); B32B 5/022 (2013.01); B32B 5/26 (2013.01); B32B 7/02 (2013.01); B32B 7/12 (2013.01); D01D 5/0007 (2013.01); D01D 5/0038 (2013.01); D01D 5/0061 (2013.01); D01D 5/0076 (2013.01); D04H 1/728 (2013.01); D04H 3/16 (2013.01); A61F 13/02 (2013.01); A61L 2300/414 (2013.01); A61L 2400/12 (2013.01); A61L 2400/18 (2013.01); A61L 2430/20 (2013.01); A61L 2430/32 (2013.01); B32B 2250/20 (2013.01); B32B 2262/0276 (2013.01); B32B 2307/518 (2013.01); B32B 2307/54 (2013.01); B32B 2307/726 (2013.01); B32B 2307/7265 (2013.01); B32B 2556/00 (2013.01); D10B 2509/00 (2013.01)] | 9 Claims |
|
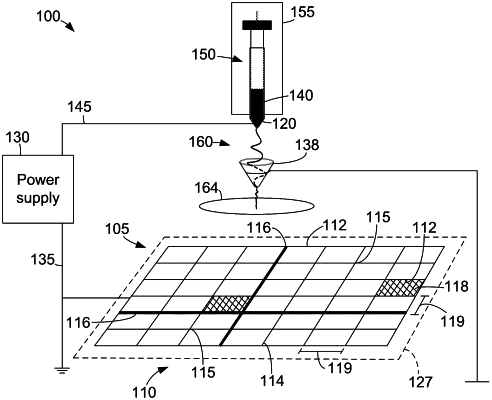
1. A three-dimensional electrospun biomedical patch for facilitating tissue repair, the three-dimensional electrospun biomedical patch comprising:
a structure of bioresorbable deposited electrospun fibers generated by electrospinning a first polymer composition and a second polymer composition, the deposited electrospun fibers extending in a plurality of directions in three dimensions to facilitate cellular migration for a period of time upon application of the three-dimensional electrospun biomedical patch to a tissue, wherein the period of time is less than twelve months, wherein the structure of deposited electrospun fibers comprise one or more high density fiber deposition areas and one or more low density fiber deposition areas, and the one or more high density fiber deposition areas and the one or more low density fiber deposition areas are entangled; and
a plurality of voids within the structure of bioresorbable deposited electrospun fibers, the plurality of voids comprising one or more voids between 10 μm and 10 cm in length,
wherein the three-dimensional electrospun biomedical patch is pliable and resistant to tearing to enable movement of the three-dimensional electrospun biomedical patch with the tissue.
|