| CPC A61K 9/1272 (2013.01) [A61K 31/7115 (2013.01)] | 16 Claims |
|
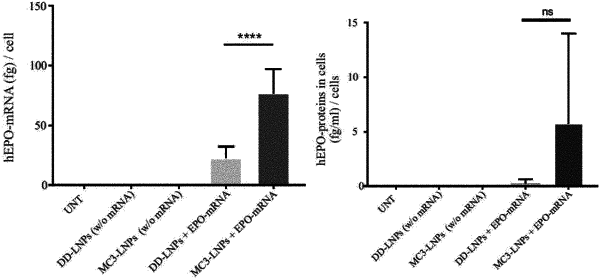
1. An isolated exosome comprising a modified ribonucleic acid (RNA) prepared by a process comprising:
(a) providing one or more lipid nanoparticles (LNP) comprising the modified RNA;
(b) contacting one or more cells with the LNP under conditions that allow LNP uptake by the one or more cells; and
(c) isolating exosomes produced by the one or more cells, wherein at least one isolated exosome comprises the modified RNA;
wherein the exosome comprises an ionizable lipid:modified RNA nucleotides molar ratio of about 1:1 to about 3:1, or less than about 1:1.
|