| CPC A61K 9/0034 (2013.01) [A61K 9/0002 (2013.01); A61K 31/4409 (2013.01); A61K 31/4422 (2013.01); A61K 45/06 (2013.01)] | 15 Claims |
|
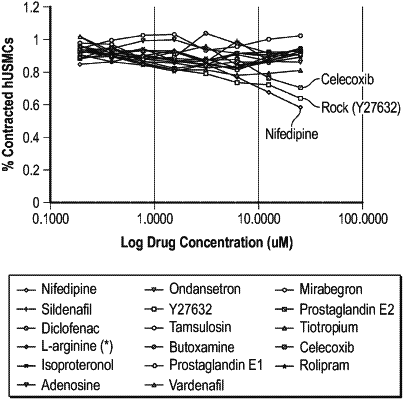
1. A formulation for local administration to a ureter of a patient, the formulation comprising:
a therapeutic agent selected from a calcium channel blocker, a rho kinase inhibitor, or a combination thereof; and
one or more pharmaceutically acceptable excipients,
wherein the formulation is suitable for local administration into the ureter, the formulation is configured to delay passage of the formulation from the ureter, and the formulation is in the form of a liquid solution or a viscous vehicle.
|