| CPC A61K 35/42 (2013.01) [A01K 67/0271 (2013.01); A61K 9/007 (2013.01); A61K 9/0073 (2013.01); A61K 45/06 (2013.01); A61K 49/0008 (2013.01); C12N 5/0062 (2013.01); C12N 5/0688 (2013.01); C12N 5/0697 (2013.01); A01K 2207/12 (2013.01); A01K 2227/105 (2013.01); A01K 2267/0337 (2013.01); C12N 2506/02 (2013.01); C12N 2506/27 (2013.01); C12N 2506/45 (2013.01); C12N 2513/00 (2013.01)] | 17 Claims |
|
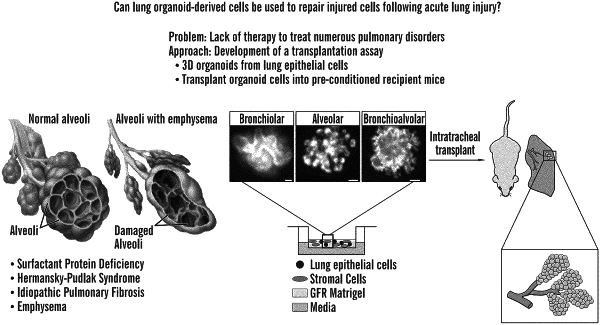
1. A method for treating a lung disease or disorder, or a lung injury, in a subject in need thereof, the method comprising administering to a subject, intratracheally, a therapeutically effective amount of a composition comprising an alveolar organoid or an isolated cell thereof,
wherein the alveolar organoid is provided or produced in vitro or ex vivo from a lung cell by culturing the lung cell in a 3-dimensional culture for a time and under conditions sufficient to produce the alveolar organoid,
wherein administering is transplanting, and
wherein the alveolar organoid or cell thereof functionally engrafts into the lung after transplanting thereby treating the lung disease or disorder or lung injury in the subject.
|