| CPC A61K 31/519 (2013.01) [A61K 8/4946 (2013.01); A61K 8/4953 (2013.01); A61K 8/4966 (2013.01); A61K 9/0019 (2013.01); A61K 31/496 (2013.01); A61K 31/4985 (2013.01); A61K 31/53 (2013.01); A61Q 19/00 (2013.01); A61Q 19/06 (2013.01); A61K 31/4162 (2013.01); C07D 487/14 (2013.01)] | 19 Claims |
|
1. A cosmetic method of modifying the contour of a subject's externally exposed body part containing fat, the method comprising administering to said body part an amount of a compound of Formula I effective to modify the contour of said body part, wherein Formula I is represented by:
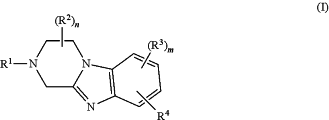 or a pharmaceutically acceptable salt thereof, wherein:
R1 is phenyl, 5-6 membered heteroaryl, aralkyl, —(C1-C6)alkyl, —(C3-C6)cycloalkyl, —(C1-C6)alkylene-OH, or —(C1-C6)alkylene-O—(C1-C6)alkyl, wherein said phenyl, 5-6 membered heteroaryl, aralkyl, and cycloalkyl are optionally substituted with 1, 2, or 3 substituents independently selected from the group consisting of halogen, —(C1-C6)alkyl, —(C3-C6)cycloalkyl, hydroxyl, —(C1-C6)alkoxy, and —N(R5)R6;
R2 and R3 each represent independently for each occurrence hydrogen, —(C1-C6)alkyl, or halogen;
R4 is —C(O)N(R5)R6, —N(R5)C(O)R6, —CO2R6, —C(O)R6, —(C1-C6)alkylene-N(R5)R6, —(C1-C6)alkylene-OR6, or —(C1-C6)alkoxy;
R5 and R6 each represent independently for each occurrence hydrogen, —(C1-C6)alkyl, or —(C3-C6)cycloalkyl; or when R5 and R6 are attached to the same nitrogen atom, then R5 and R6 may be taken together with the nitrogen atom to which they are attached to form a 3-7 membered heterocycle; and
n and m each represent independently 1 or 2.
|