| CPC A61F 2/04 (2013.01) [A61F 2002/044 (2013.01); A61F 2210/0004 (2013.01)] | 20 Claims |
|
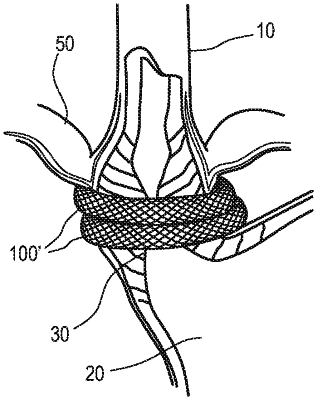
1. A bioabsorbable scaffold, comprising:
a matrix comprising:
a first bioabsorbable filament; and
a second bioabsorbable filament,
wherein the matrix is configured to be disposed at least partially around a body portion or a sphincter of a patient,
wherein the matrix is configured to induce a formation of collagen tissue about a portion of the sphincter,
wherein the first bioabsorbable filament has a first bioabsorption rate and the second bioabsorbable filament has a second bioabsorption rate that is different from the first bioabsorption rate, and
wherein the matrix is configured to couple the body portion or the sphincter of the patient to an adjacent body portion.
|