| CPC G16H 40/63 (2018.01) [A61B 1/00006 (2013.01); A61B 1/00059 (2013.01); A61B 1/043 (2013.01); A61B 1/045 (2013.01)] | 20 Claims |
|
1. A method of operating a medical system that is operable within at least two different modes, the method comprising:
receiving an indicator for performing at least one medical procedure that utilizes a medical device;
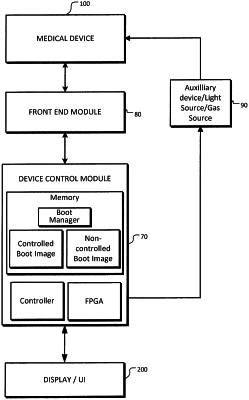
based on the indicator, selecting one of multiple binary images for booting a medical device control module, the multiple binary images including at least a controlled type binary image, for which updates are controlled by an external authority, for operating as a first medical device and a non-controlled type binary image for operating as a second medical device;
if the selected binary image is the controlled type binary image, booting the medical device control module from the controlled type binary image and subsequently operating the medical device control module as the first medical device for performing the at least one medical procedure;
if the selected binary image is a non-controlled type binary image, booting the medical device control module from the non-controlled binary image and subsequently operating the medical device control module as the second medical device for performing the at least one medical procedure,
wherein the first medical device is an FDA Class III medical device, according to the Class III regulation at the time of filing of the priority patent application for this invention, that is Mar. 2, 2017; and the second medical device is one of an FDA Class I and Class II device according to the Class I and Class II regulations at the time of filing of the priority patent application for this invention, that is Mar. 2, 2017;
updating a selected non-controlled binary image; and
subsequently, recognizing that a different medical device has been coupled to a medical device control module, obtaining a device identifier for the different medical device, and based on the device identifier, selecting a replacement binary image and then booting the medical device control module from the replacement binary image.
|