| CPC G16H 40/20 (2018.01) [G06Q 10/06398 (2013.01); G06Q 10/063116 (2013.01); G06Q 30/018 (2013.01); G09B 19/003 (2013.01); G16H 10/60 (2018.01); G16H 20/17 (2018.01); G16H 40/60 (2018.01); G16H 50/20 (2018.01); G16H 70/20 (2018.01); H04L 63/08 (2013.01); G06F 3/14 (2013.01)] | 22 Claims |
|
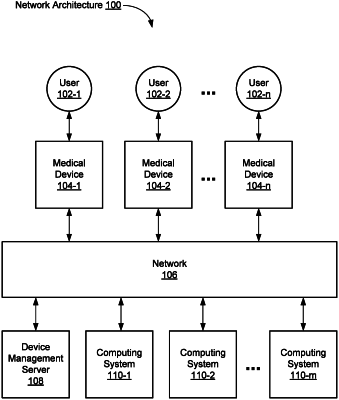
1. A method performed by one or more computing devices, comprising:
identifying interactions of a first user with a plurality of medical devices in a healthcare organization, one of the interactions including a response time to one or more alarms generated by a respective medical device of the plurality of medical devices, the plurality of medical devices including an infusion device, a syringe pump, a medication preparation workstation, or an automated medication dispensing cabinet;
determining, based on the identified interactions and a predetermined set of rules, a compliance score associated with the first user;
in response to the compliance score not satisfying a threshold compliance score, automatically, without user involvement:
transmitting an instruction to at least one medical device of the plurality of medical devices to cause an access level of the first user to the at least one medical device to be reduced by denying access to a number of respective features of the at least one medical device that are available to the first user;
generating a training program associated with the at least one medical device;
sending a training package associated with the training program to the first user and notifying the first user to complete the training program using the training package, and
restoring the access level of the first user with regard to the at least one medical device responsive to the first user completing the training program.
|