| CPC C12N 9/1025 (2013.01) [C12P 17/06 (2013.01); C12Y 203/01174 (2013.01)] | 13 Claims |
|
1. A method of making a polyketide, comprising growing a genetically engineered microorganism in a nutrient broth for a time sufficient to produce a polyketide and isolating said polyketide or a spontaneously rearranged form of said polyketide or a derivative of said polyketide, said polyketide selected from triacetic acid lactone, dehydroacetic acid, olivetolic acid, orsellinic acid, or 6-methylsalicylic acid and said derivative of said polyketide is a prenylated aromatic, or a cannabinoid, or dehydroacetic acid, or olivetolic acid, or cannabigerolic acid, or orsellinic acid, or 6-methylsalicylic acid, wherein said microorganism has a polyketide-producing pathway comprising the following substrate(s) to product(s) conversions:
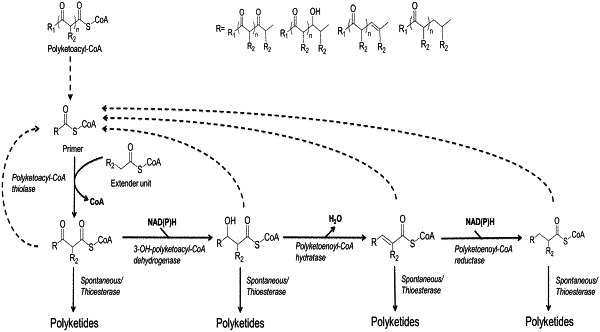
a) C(n)-acyl-CoA+acetyl-CoA→C(n+2)-ketoacyl-CoA catalyzed by a ketoacyl-CoA thiolase encoded by Streptomyces collinus fadA, Rhodococcus opacus pcaF, Pseudomonas putida pcaF, Streptomyces sp. pcaF, P. putida fadAx, P. putida fadA, Acinetobacter sp. ADP1 dcaF, or Ralstonia eutropha bktB;
b) C(n+2)-ketoacyl-CoA+acetyl-CoA→C(n+4)-polyketoacyl-CoA catalyzed by a polyketoacyl-CoA thiolase encoded by Streptomyces collinus fadA, Rhodococcus opacus pcaF, Pseudomonas putida pcaF, Streptomyces sp. pcaF, P. putida fadAx, P. putida fadA, Acinetobacter sp. ADP1 dcaF, or Ralstonia eutropha bktB;
c) optionally, C(n+4)-polyketoacyl-CoA→3-OH—C(n+4)-polyketoacyl-CoA;
d) optionally, 3-OH—C(n+4)-polyketoacyl-CoA→C(n+4)-polyketoenoyl-CoA; and
e) optionally, C(n+4)-polyketoenoyl-CoA→C(n+4)-α,β-saturated-polyketoacyl-CoA;
f) iterations of at least one of the reactions in steps b), c), d), and e) wherein said iterations are achieved by utilizing a product generated in reactions steps b), c), d), and e), respectively, as a substrates for condensation with acetyl-CoA to elongate said product by two carbons and add a beta-keto group; and
g) conversion of said product formed in steps b), c), d), e), or a product formed in step f) to a polyketide or a spontaneously rearranged form of said polyketide or a derivative of said polyketide;
wherein n>0 and <30.
|