| CPC C09K 19/46 (2013.01) [C07C 69/92 (2013.01); C09K 19/3857 (2013.01); C09K 19/56 (2013.01); C09K 19/60 (2013.01); G02F 1/1337 (2013.01); C09K 2019/0448 (2013.01)] | 15 Claims |
|
1. A mesogen-containing compound represented by the following Formula (I),
(Mesogen-1)-L1-(Mesogen-2)-L2-(Mesogen-3) (I)
wherein
(A) Mesogen-1 and Mesogen-3 are each independently represented by the following Formula (II),
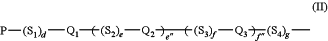 wherein for Formula (II),
P is selected from hydrogen, halogen, alkyl, haloalkyl, alkoxy, haloalkoxy, acrylate, methacrylate, trihalomethacrylate, cyanoacrylate, acrylamido, methacrylamido, oxirane, hydroxyl, primary amino, carboxylic acid, or carboxylic acid ester;
(B) Mesogen-2 is represented by the following Formula (III),
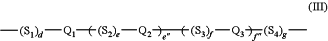 wherein independently for each of Formula (II) and Formula (III)
S1, S2, S3, and S4, for each occurrence, are independently selected from a spacer unit chosen from —CH2—; —O—; —C(O)—; —N═N—; —CH═CH—; —C≡C—; —CH═N—; —CF2—; or —NH—, provided that when two spacer units comprising heteroatoms are linked together the spacer units are linked so that heteroatoms are not directly linked to each other;
d is 0 to 20;
e, f, and g, for each occurrence, are independently 0 to 3;
Q1, Q2, and Q3, for each occurrence, are independently a divalent group selected from the group consisting of unsubstituted or substituted cycloaliphatic group; unsubstituted or substituted heterocycloaliphatic group; unsubstituted or substituted aryl; and unsubstituted or substituted heteroaryl; wherein the cycloaliphatic group substituents, heterocycloaliphatic group substituents, aryl substituents, and heteroaryl substituents are each independently selected from cyano or —(S1)d—P, where S1, d, and P are each as defined with regard to Formula (II); and
e″ and f″, for each occurrence, are independently from 0 to 6, provided the sum of e″ and f″ is at least 1; and
(C) -L1- and -L2- are each independently represented by the following Formula (IV),
-(A-B)y-E- (IV)
wherein
(i) y is 0 to 30;
(ii) each A independently for each y is a divalent group selected from the group consisting of aliphatic group and haloaliphatic group;
(iii) each B independently for each y is a divalent group selected from the group consisting of —O—; —C(O)O—; —OC(O)O—; —C(O)N(R1)— where R1 is H or alkyl;
—NH—C(O)O—; —N(R2)C(O)N(R2)— where each R2 is independently selected from H or alkyl;
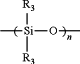 where n is 1 to 5, and each R3 independently for each n is selected from methyl, ethyl, or phenyl; and —Si(R4)(R4)— where each R4 is independently selected from methyl, ethyl, or phenyl; and
(iv) E is a divalent group selected from the group consisting of aliphatic group and haloaliphatic group,
provided that at least one of Mesogen-1, Mesogen-2, or Mesogen-3, include at least four cyclic groups, and
provided that -L1- and -L2- each independently comprise an average chain length of at least 20 bonds.
|