| CPC C07F 5/04 (2013.01) [C07D 213/64 (2013.01); C07D 213/68 (2013.01); C07D 213/69 (2013.01); C07D 239/30 (2013.01); C07D 239/47 (2013.01); C07D 241/04 (2013.01); C07D 401/04 (2013.01); C07D 401/14 (2013.01); C07D 413/04 (2013.01); C07D 413/14 (2013.01); C07D 417/12 (2013.01); C07D 417/14 (2013.01); A61K 45/06 (2013.01)] | 17 Claims |
|
1. A process for preparing a compound represented by Formula (I):
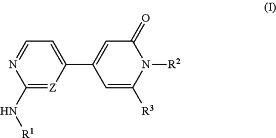 wherein:
R1 is phenyl or monocyclic 5-6 membered heteroaryl, each optionally substituted with one or more substituents selected from halogen, C1-C6alkyl, C3-C4cycloalkyl, C1-C6alkoxy, C1-C6haloalkyl, C1-C6haloalkoxy, amino, N—C1-C3alkylamino and N,N-diC1-C3alkylamino;
R2 is selected from the group consisting of hydrogen, C1-C3haloalkyl and C1-C3alkyl;
R3 is selected from the group consisting of A, phenyl and monocyclic heteroaryl, said phenyl and said heteroaryl being each optionally substituted with one or more of R4, R5, R6 and R7;
R4, R5, R6 and R7 are independently selected from halogen, C1-C6alkyl, C3-C4cycloalkyl, C1-C6alkoxy, C1-C6haloalkyl, C1-C6haloalkoxy, azetidine, amino, N—C1-C3alkylamino, N,N-diC1-C3alkylamino, NHSO2R8, SO2R9 and hydroxy;
R8 is C1-C3haloalkyl or C1-C3alkyl;
R9 is selected from the group consisting of R10, C1-C6alkyl, amino, N—C1-C3alkylamino, N,N-diC1-C3alkylamino and C1-C3alkoxyC1-C3alkyl, wherein said C1-C6alkyl and C1-C3alkoxyC1-C3alkyl being each optionally substituted with one R10 and/or one or more halogen;
R10 is selected from the group consisting of phenyl, benzyl, monocyclic heteroaryl, C3-C6cycloalkyl, heterocyclyl, each optionally substituted with one or more R11;
R11 is selected from the group consisting of halogen, C1-C3haloalkyl, C3-C4cycloalkyl, C1-C3alkyl, amino, N—C1-C3alkylamino, N,N-diC1-C3alkylamino and C1-C3alkoxyC1-C3alkyl;
A is
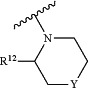 R12 is selected from the group consisting of hydrogen, halogen, COR13, C1-C6alkyl, C3-C6cycloalkyl, C1-C3alkoxyC1-C3alkyl, C1-C6alkoxy, C3-C6cycloalkyl, C1-C3cyanoalkyl, and C1-C3haloalkyl;
R13 is selected from the group consisting of C1-C3alkoxy, N—C1-C3alkylamino, N,N-diC1-C3alkylamino, 1-pyrrolidinyl, 1-piperidinyl and 1-azetidinyl;
Y is selected from the group consisting of CH2, S, SO, SO2, NR14, NCOR9, NCOOR15, NSO2R9, NCOCH2R9, O, or a bond;
R14 is selected from the group consisting of H, C1-C3haloalkyl, C1-C3alkoxyC1-C3alkyl, C1-C3alkyl, and C3-C6cycloalkyl;
R15 is selected from the group consisting of R10, C1-C6alkyl and C1-C3alkoxyC1-C3alkyl, and wherein said C1-C6alkyl and C1-C3alkoxyC1-C3alkyl being each optionally substituted with one R10 and/or one or more halogen; and
Z is CH or N;
or a pharmaceutically acceptable salt thereof;
the process comprising:
(i) providing a compound represented by Formula (III):
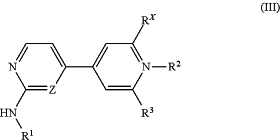 wherein:
R1, R2, R3, and Z are defined as above for Formula (I); and
RX is selected from the group consisting of F, OCH3, OC(CH3)3, and OSiR′R′R′″; and
R′, R″, and R′″ are each independently aryl or alkyl; and
(ii) converting the compound represented by Formula (III) into the compound represented by Formula (I).
|