| CPC C07D 471/04 (2013.01) [C07D 403/14 (2013.01); C07B 2200/13 (2013.01)] | 72 Claims |
|
1. A process for preparing a compound of formula (VIII) or a salt thereof, the process comprising:
(a) reacting a reaction mixture comprising a compound of formula (IV), a compound of formula (V) or a compound of formula (X), and an organic solvent to form a compound of formula (VI) according to step 1 below
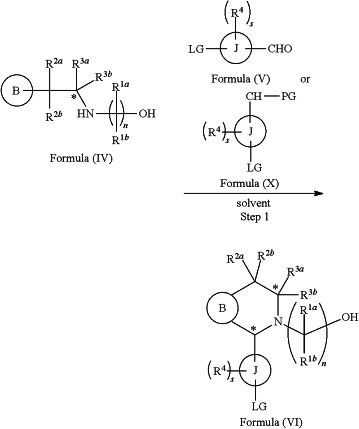 wherein
B is substituted or unsubstituted indolyl, benzofuranyl, benzothiophenyl, aza-indolyl, indazolyl, benzimidazolyl, pyrrolopyridinyl, furopyridinyl, thienopyridinyl, pyrrolopyridazinyl, pyrrolopyrimidinyl, pyrrolopyrazinyl, thienopyridazinyl, thienopyrimidinyl, thienopyrazinyl, furopyridazinyl, furopyrimidinyl, or furopyrazinyl;
each of R1a and R1b is independently hydrogen, fluorine, chlorine, —OH, C1-3 alkyl, C1-3 haloalkyl, C1-3 alkoxy, C1-3 hydroxyalkyl, and —CN, C3-6 cycloalkyl, or C3-6 spirocycloalkyl,
n is an integer of 2 or 3,
each of R2a and R2b is independently hydrogen, halogen, —OH, C1-3 alkyl, C1-3 haloalkyl, C1-3 alkoxy, C1-3 hydroxyalkyl, —CN, C3-6 cycloalkyl, or C3-6 spirocycloalkyl,
R3a and R3b are independently hydrogen, C1-3 alkyl, C1-3 haloalkyl, C1-3 alkoxy, —CN, C3-6 cycloalkyl, C3-6 heterocycloalkyl, phenyl, C3-6 heteroaryl, or C3-6 spirocycloalkyl,
J is phenyl or pyridinyl;
each R4 is independently hydrogen, halogen or C1-3 alkyl,
s is an integer from 0 to 2,
LG is a leaving group,
LG and CHO are located in the para position with respect to each other on J on the compound of formula (V),
PG is an aldehyde protecting group,
LG and CH-PG are located in the para position with respect to each other on J on the compound formula (X), and
each asterisk independently represents a chiral center wherein the carbon bearing R3a and R3b is a chiral center when R3a and R3b are different; and
(b) reacting a reaction mixture comprising the compound of formula (VI), an organic solvent, and a compound of formula (VII) or a salt thereof to form a compound of formula (VIII) or a salt thereof according to step 2 below
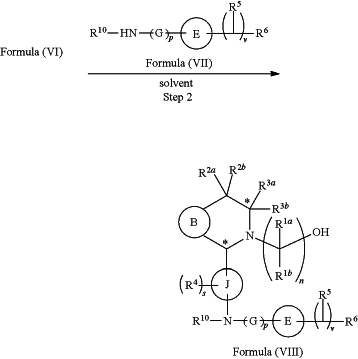 wherein
G is C1-3 alkyl,
p is 0 or 1,
E is substituted or unsubstituted azetidinyl or pyrrolidinyl,
each R5 is independently hydrogen, halogen, —OH, —CN, C1-5 alkoxy, or C1-5 hydroxyalkyl,
v is an integer from 1 to 5, and
R6 is halogen or —CN;
R10 is hydrogen or C1-3 alkyl.
|