| CPC B01J 31/2414 (2013.01) [B01J 31/2208 (2013.01); B01J 31/2273 (2013.01); B01J 31/2278 (2013.01); B01J 31/2295 (2013.01); B01J 31/2404 (2013.01); B01J 31/2409 (2013.01); C07F 15/0046 (2013.01); B01J 2231/543 (2013.01); B01J 2531/821 (2013.01)] | 2 Claims |
|
1. A method of synthesizing an olefin metathesis catalyst represented by the structure of Formula (C)
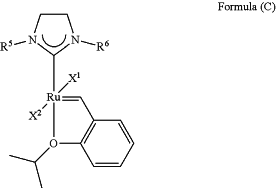 the method comprising contacting an olefin metathesis catalyst represented by the structure of Formula (IVa)
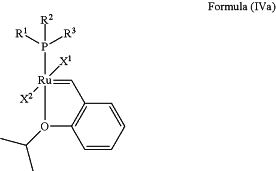 with a N-Heterocyclic Carbene ligand of formula
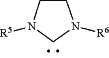 at, wherein:
X1 and X2 are independently halogen, trifluoroacetate, per-fluorophenols or nitrate;
R1 is unsubstituted saturated N-heterocycle, substituted saturated N-heterocycle, —NH(C1-C24 alkyl), —N(C1-C24 alkyl)2, —NH(C5-C24 aryl), —N(C5-C24 aryl)2, —N(C1-C24 alkyl)(C5-C24 aryl) or —NRC1-C6 alkylene)(C5-C24 aryl)]2;
R2 is unsubstituted (C5-C24 aryl), substituted (C5-C24 aryl), unsubstituted saturated N-heterocycle, substituted saturated N-heterocycle, —NH(C1-C24 alkyl), —N(C1-C24 alkyl)2, —NH(C5-C24 aryl), —N(C5-C24 aryl)2, —N(C1-C24 alkyl)(C5-C24 aryl) or —N[(C1-C6 alkylene)(C5-C24 aryl)]2;
R3 is unsubstituted (C5-C24 aryl), substituted (C5-C24 aryl), unsubstituted saturated N-heterocycle or substituted saturated N-heterocycle;
R5 and R6 are independently hydrogen, unsubstituted C5-C24 aryl, or substituted C5-C24 aryl;
generally R5 and R6 are independently substituted C5-C24 aryl with one to three unsubstituted (C1-C6 alkyl) groups or substituted (C1-C6 alkyl) groups.
|