| CPC C09D 183/08 (2013.01) [B01J 27/125 (2013.01); B01J 27/128 (2013.01); B01J 31/08 (2013.01); B01J 31/30 (2013.01); C07F 7/1804 (2013.01); C08G 77/442 (2013.01); C08G 77/46 (2013.01); C09C 1/3081 (2013.01); C09D 183/12 (2013.01); C08G 77/70 (2013.01)] | 1 Claim |
|
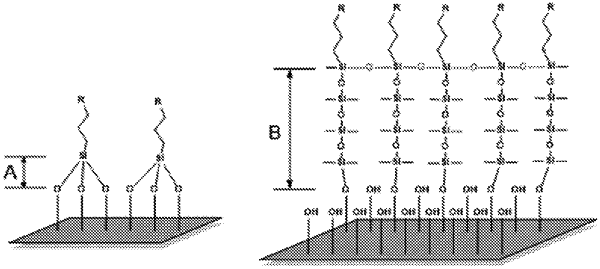
1. A method for the treatment of fillers comprising:
1) applying a silane coupling agent and drying at room temperature;
2) applying a functionalized hydrophilic fumed silica with a specific surface area of 200 m2/g and drying at room temperature;
3) applying an organofunctional siloxane selected from the group consisting of:
an organofunctional siloxane having the formula (V)
XR1SiR2(OSiR2)n+1OSiR3 (V)
wherein
X is selected from the group consisting of hydrogen, hydroxyl, aldehyde, acetate, amino, alkylimine, alkylamino, dialkylamino, diaminoalkyl, ureido, isocyanate, anhydride, epoxycyclohexyl, glycidoxy, mercapto, acryloxy, methacryloxy, methacrylamide, acrylamide, alkylamide, carboxylic acid, alkenyl, 2-hydroxy-3-(methacryloyloxy)propan-l-olate, alkyl, cycloalkyl, aryl, alkylaryl, arylalkyl, aryloxy, perfluoroalkyl, perfluoroaryl, alkylpolyalkylenoxy and hydroxypolyalkylenoxy;
R is selected from the group consisting of halogen, hydrogen, hydroxyl, alkyl, alkenyl, alkynyl, cycloalkyl, aryl, arylalkyl, alkylaryl and dialklyaryl, and alkoxy, acyloxy, alkyloyl, carbinoyl acyloxy group, alkylamine, dialkylamine, oximino and enoxy;
R1is selected from the group consisting of methyl, ethyl, propyl, butyl, isobutyl, phenyl and phenethyl; and
n is an integer having a value of 2 to 100,
an organofunctional siloxane having the formula (VII)
R3SiO(R2SiO)nSiR2R2SiR2(OSiR2)nOSiR3 (VII)
wherein
R is selected from the group consisting of hydrogen, hydroxyl, alkyl, alkenyl, alkynyl, cycloalkyl, aryl, arylalkyl, alkylaryl, dialklyaryl, alkoxy, acyloxy, alkyloyl, carbinoyl acyloxy group, alkylamine, dialkylamine, oximino and enoxy;
R2 is selected from the group consisting of methyl, ethyl, propyl, butyl, isobutyl, phenyl and phenethyl; and
n is an integer having a value of 2 to 100, and
an organofunctional siloxane having the formula (VIII)
R3SiO(R2SiO)n(SiR2)R1SyR1(SiR2)(OSiR2)nOSiR3 (VIII)
wherein
R is selected from the group consisting of hydrogen, hydroxyl, alkyl, alkenyl, alkynyl, cycloalkyl, aryl, arylalkyl, alkylaryl, dialklyaryl, alkoxy, acyloxy, alkyloyl, carbinoyl acyloxy group, alkylamine, dialkylamine, oximino and enoxy;
R1 is selected from the group consisting of alkyl, cycloalkyl, aryl, arylalkyl, alkylaryl and dialklyaryl;
n is an integer having a value of 2 to 100; and
y is an integer having a value of 1 to 100,
wherein the fillers are quartz, silica, glass, aluminium, clays, silicon, copper, tin, talc, inorganic oxides, steel, asbestos, nickel, zinc, lead, marble, gypsum, graphite or carbon.
|