| CPC C07H 15/207 (2013.01) [C25B 3/25 (2021.01); C25B 9/19 (2021.01)] | 43 Claims |
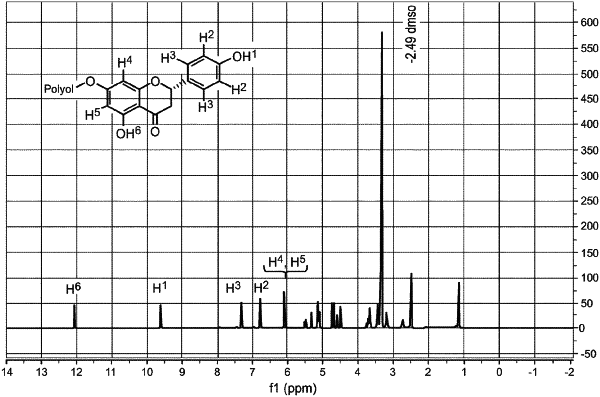
|
1. A method of forming a dihydrochalcone, the method comprising:
electrocatalytically hydrogenating (ECH) a reactant compound over a catalytic cathode in a reaction medium having a non-alkaline pH value, thereby forming a dihydrochalcone product; wherein:
the reactant compound has a structure according to Formula (I):
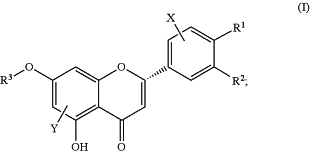 each of R1 and R2 is independently selected from the group consisting of H and OH, with the proviso that at least one of R1 and R2 is OH;
R3 is selected from the group consisting of H, C1-3 alkyl, C1-3 alkylene-NRaRb, C0-3 alkylene-PO3H2, C1-4 alkylene-SO3H, C1-3 alkylene-CO2H, C1-5 alkylene-OH, and a saccharide moiety, wherein each alkylene is optionally substituted with one or more of —CO2H, —NH2, —OH, and —SO3H;
each Ra and Rb is independently selected from the group consisting of H and SO3H;
with a further proviso that R1, R2, and R3 are selected from the group consisting of (a), (b), (c), (d), and (e) in which:
(a) R1 is H, R2 is as defined above, and R3 is as defined above;
(b) R2 is H, R1 is as defined above, and R3 is as defined above;
(c) R1 is OH, R2 is H, and R3 is H or the saccharide moiety;
(d) R1 is OH, R2 is H, and R3 is CH3 or the saccharide moiety; and
(e) R1 is OH, R2 is OH, and R3 is H or the saccharide moiety;
X is selected from the group consisting of H, OH, C1-3 alkyl, NH2, halo, and C1-3 alkoxy;
Y is selected from the group consisting of H and C1-3 alkyl; and,
the dihydrochalcone product has a structure according to Formula (II):
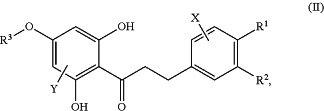 in which R1, R2, R3, X, and Y are as defined for Formula (I).
|