| CPC C07F 7/10 (2013.01) | 5 Claims |
|
1. A method of preparing a compound of Formula (IA) or (IIA):
 wherein
R1 is H, an optionally substituted alkyl, optionally substituted alkylene, optionally substituted alkenyl, optionally substituted alkenylene, optionally substituted alkynyl, optionally substituted alkynylene, optionally substituted aryl, optionally substituted arylene, optionally substituted heteroalkyl, optionally substituted heteroalkylene, optionally substituted heteroaryl, optionally substituted heteroarylene, or optionally substituted metallocene; and
R2 is an optionally substituted aryl or an optionally substituted heteroaryl group;
wherein R1 is optionally linked with R2 to form a cyclic moiety such that R1 is the optionally substituted alkylene, optionally substituted alkenylene, optionally substituted alkynylene, optionally substituted arylene, optionally substituted heteroalkylene, optionally substituted heteroarylene, or optionally substituted aralkylene;
[Si] is —(R)2Si—X, where X is H, 19F, or 18F, and
R is independently an optionally substituted C1-12 alkyl, an optionally substituted C1-12 heteroalkyl group, and optionally substituted aryl group, or an optionally substituted heteroaryl group, and
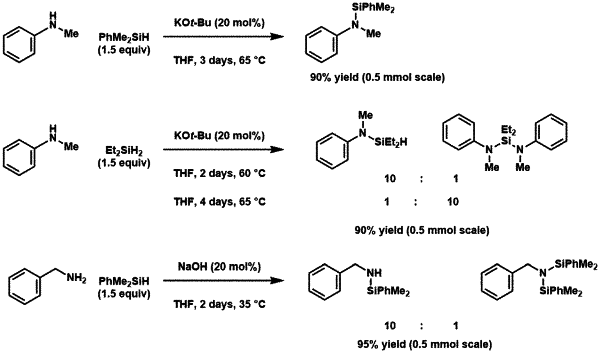
wherein the optional substituents in R1 and R2 are independently halo, optionally protected hydroxyl, sulfhydryl, C1-C24 alkyl, C2-C24 alkenyl, C2-C24 alkynyl, C1-C24 alkoxy, C2-C24 alkenyloxy, C2-C24 alkynyloxy, C5-C24 aryl, C6-C24 alkaryl, C6-C24 aralkyl, C5-C24 aryloxy, C6-C24 aralkyloxy, C6-C24 alkaryloxy, C1-C24 alkylcarbonyl, C6-C24 arylcarbonyl, C2-C24 alkylcarbonyloxy, C6-C24 arylcarbonyloxy, C2-C24 alkoxycarbonyl, C6-C24 aryloxycarbonyl, C2-C24 alkylcarbonato, C6-C24 arylcarbonato, carbamoyl, mono-(C1-C24 alkyl)-substituted carbamoyl, di-(C1-C24 alkyl)-substituted carbamoyl, mono-(C5-C24 aryl)-substituted carbamoyl, di-(C5-C24 aryl)substituted carbamoyl, N—(C1-C24 alkyl), N—(C5-C24 aryl)-substituted carbamoyl, optionally protected carboxylato, thiocarbamoyl, mono-(C1-C24 alkyl)-substituted thiocarbamoyl, di-(C1-C24 alkyl)-substituted thiocarbamoyl, mono-(C5-C24 aryl)substituted thiocarbamoyl, di-(C5-C24 aryl)-substituted thiocarbamoyl, N—(C1-C24 alkyl),N—(C5-C24 aryl)-substituted thiocarbamoyl, carbamido, cyano, cyanato, thiocyanato, thioisocyanate, formyl, thioformyl, optionally protected amino, mono-(C1-C24 alkyl)-substituted amino, di-(C1-C24 alkyl)-substituted amino, mono-(C5-C24 aryl)substituted amino, di-(C5-C24 aryl)-substituted amino, C2-C20 alkylimino, arylimino, mercapto, nitro, nitroso, sulfo, sulfonato, C1-C24 alkylsulfanyl, C5-C24 arylsulfanyl, C1-C24 alkylsulfinyl, C5-C24 arylsulfinyl, C1-C24 alkylsulfonyl, C1-C24 monoalkylaminosulfonyl, C1-C24 dialkylaminosulfonyl, C1-C24 aryl sulfonyl, oligopeptide, peptide, polypeptide, and/or polyglycol, the method comprising forming an N—Si bond by contacting an organic substrate comprising an aromatic-substituted amine having at least one N—H bond with a mixture comprising of (a) at least one hydrosilane and (b) at least one sodium hydroxide, potassium hydroxide, sodium alkoxide, or potassium alkoxide, under conditions sufficient to form the N—Si bond, wherein the mixture is free of added transition-metal species and the aromatic-substituted amine having at least one N—H bond has a structure of Formula (I) or Formula (II):
 where
R1 is H, an optionally substituted alkyl, optionally substituted alkylene, optionally substituted alkenyl, optionally substituted alkenylene, optionally substituted alkynyl, optionally substituted alkynylene, optionally substituted aryl, optionally substituted arylene, optionally substituted heteroalkyl, optionally substituted heteroalkylene, optionally substituted heteroaryl, optionally substituted heteroarylene, or optionally substituted metallocene; and
R2 is an optionally substituted aryl or an optionally substituted heteroaryl group; and
wherein R1 is optionally linked with R2 to form a cyclic moiety such that R1 is the optionally substituted alkylene, optionally substituted alkenylene, optionally substituted alkynylene, optionally substituted arylene, optionally substituted heteroalkylene, optionally substituted heteroarylene, or optionally substituted aralkylene;
wherein the at least one hydrosilane comprises a compound of Formula (I):
(R)3-mSi(H)m+1 (I)
where m is 1; and
each R is independently optionally substituted C1-12 alkyl, an optionally substituted C1-12 heteroalkyl, an optionally substituted C6-20 aryl, or an optionally substituted C5-20 heteroaryl, and, if substituted, the substituents are independently optionally protected amino, amido, nitro, nitroso, optionally protected hydroxyl, C1-20 alkoxy, C6-20 aryloxy, C2-20 alkoxycarbonyl, C5-20 aryloxycarbonyl, carboxyl, optionally protected carboxylato, mercapto, formyl, C1-20 thioester, cyano, cyanato, thiocyanato, isocyanate, thioisocyanate, carbamoyl, epoxy, or silyl
to form the compound of Formula (IA) or (IIA), wherein X is H.
|