| CPC C07D 475/00 (2013.01) | 13 Claims |
|
1. A compound of Formula (IA) or a salt, solvate, enantiomer, diastereoisomer, or tautomer thereof:
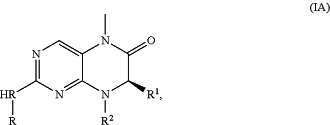 wherein:
R is
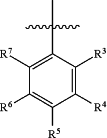 or heteroaryl,
wherein the heteroaryl is optionally substituted with at least one selected from the group consisting of C1-C6 alkyl, C3-C8 cycloalkyl, C1-C6 alkoxy, C3-C8 cycloalkoxy, halogen, —OH, —C(═O)OH, —C(═O)C1-C6 alkyl, —C(═O)C3-C8 cycloalkyl, thiol, C1-C6 thioalkoxy, and —NR′R′,
wherein each occurrence of R′ is independently H or C1-C6 alkyl or the two R′ bound to the N combine to form 3-7 membered heterocyclyl;
R1 is selected from the group consisting of isopropyl, sec-butyl, tert-butyl, C3-C8 cycloalkyl, C1-C6 heteroalkyl, C3-C8 heterocycloalkyl,
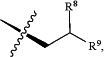 and C1-C6 alkyl substituted with at least one group selected from C3-C8 cycloalkyl, C1-C6 alkoxy, C3-C8 cycloalkoxy, halogen, —OH, thiol, C1-C6 thioalkoxy, phenyl, heteroaryl, heterocyclyl, and —NR″R″,
wherein in R1 each occurrence of cycloalkyl, heteroalkyl, or heterocycloalkyl is optionally substituted with at least one substituent selected from the group consisting of C1-C6 alkyl, C3-C8 cycloalkyl, C1-C6 alkoxy, C3-C8 cycloalkoxy, halogen, —OH, thiol, C1-C6 thioalkoxy, phenyl, heteroaryl, heterocyclyl, and —NR″R″;
each occurrence of R″ is independently H or C1-C6 alkyl or the two R″ bound to the N combine to form 3-7 membered heterocyclyl;
R2 is selected from the group consisting of C1-C6 alkyl, C3-C8 cycloalkyl, C1-C6 heteroalkyl, and C3-C8 heterocycloalkyl;
wherein in R2 each occurrence of alkyl, cycloalkyl, heteroalkyl, or heterocycloalkyl is optionally substituted with at least one substituent selected from the group consisting of C1-C6 alkyl, C3-C8 cycloalkyl, C1-C6 alkoxy, C3-C8 cycloalkoxy, halogen, —OH, thiol, C1-C6 thioalkoxy, phenyl, heteroaryl, heterocyclyl, and —NR′″R′″,
wherein each occurrence of R′″ is independently H or C1-C6 alkyl or the two R′″ bound to the N combine to form 3-7 membered heterocyclyl;
R3 is selected from the group consisting of H, halogen, C1-C6 alkyl, and C3-C8 cycloalkyl;
R4 is selected from the group consisting of H, halogen, C1-C6 alkyl, C3-C8 cycloalkyl, —OH, —C(═O)OH, —C(═O)C1-C6 alkyl, and —C(═O)C3-C8 cycloalkyl;
R5 is selected from the group consisting of H, —OH, —C(═O)OH, —C(═O)C1-C6 alkyl, and —C(═O)C3-C8 cycloalkyl;
R6 is selected from the group consisting of H, halogen, C1-C6 alkyl, C3-C8 cycloalkyl, —OH, —C(═O)OH, —C(═O)C1-C6 alkyl, and —C(═O)C3-C8 cycloalkyl;
R7 is selected from the group consisting of H, halogen, C1-C6 alkyl, and C3-C8 cycloalkyl;
R8 is selected from the group consisting of C1-C6 alkyl, C3-C8 cycloalkyl, halogen, thiol, C1-C6 thioalkoxy, —OH, C1-C6 alkoxy, and —NR″R″; and
R9 is selected from the group consisting of H, C1-C6 alkyl, C3-C8 cycloalkyl, halogen, thiol, C1-C6 thioalkoxy, —OH, C1-C6 alkoxy, and —NR″R″,
wherein, if R9 is H, R8 is not methyl.
|