| CPC C07D 405/14 (2013.01) | 20 Claims |
|
1. A compound of Formula (I):
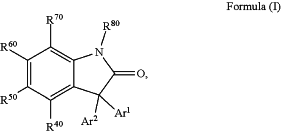 or enantiomers, prodrugs, and pharmaceutically acceptable salts thereof,
wherein:
each of R40, R50, R60, R70, and R80 independently are hydrogen, halogen, hydroxyl, amino, alkylamino, dialkylamino, acylamino, thiol, alkylthio, cyano, carbonyl, carboxyl, alkoxycarbonyl, nitro, acyl, acyloxy, alkyl, haloalkyl, heteroalkyl, alkoxy, alkenyl, alkynyl, sulfinyl, sulfonyl, thiocarbonyl, carbamoyl, cycloalkyl, heterocyclyl, aryl or heteroaryl, provided that at least one of R40, R50, R60, R70, and R80 is not H; and
Ar1 and Ar2 are each independently aryl or heteroaryl; and provided that
(i) at least one of Ar1 and Ar2 is of the structure (Ar′):
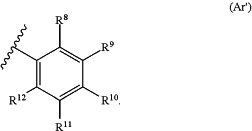 wherein:
each R8 independently is halogen, amino, alkylamino, dialkylamino, acylamino, thiol, alkylthio, cyano, carbonyl, carboxyl, alkoxycarbonyl, nitro, acyl, acyloxy, alkyl, haloalkyl, heteroalkyl, alkoxy, alkenyl, alkynyl, sulfinyl, sulfonyl, thiocarbonyl, carbamoyl, cycloalkyl, heterocyclyl, aryl or heteroaryl;
each of R9, R10, R11 and R12 independently are hydrogen, halogen, hydroxyl, amino, alkylamino, dialkylamino, acylamino, thiol, alkylthio, cyano, carbonyl, carboxyl, alkoxycarbonyl, nitro, acyl, acyloxy, alkyl, haloalkyl, heteroalkyl, alkoxy, alkenyl, alkynyl, sulfinyl, sulfonyl, thiocarbonyl, carbamoyl, cycloalkyl, heterocyclyl, aryl or heteroaryl, and each of which can be optionally substituted; or
at least a vicinal pair formed from selecting two of R9, R10, R11, or R12 and the carbons to which they are attached form an optionally substituted 5- or 6-member cycloalkyl or an optionally substituted 5- or 6-member heterocyclyl, and the remaining R9, R10, R11 or R12 independently are hydrogen, halogen, hydroxyl, amino, alkylamino, dialkylamino, acylamino, thiol, alkylthio, cyano, carbonyl, carboxyl, alkoxycarbonyl, nitro, acyl, acyloxy, alkyl, haloalkyl, heteroalkyl, alkoxy, alkenyl, alkynyl, sulfinyl, sulfonyl, thiocarbonyl, carbamoyl, cycloalkyl, heterocyclyl, aryl or heteroaryl, and each of which can be optionally substituted;
or
(ii) Ar1 and Ar2 are independently of the structure (Ar′):
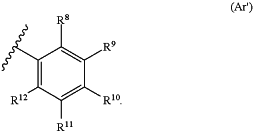 wherein:
each R8 independently is halogen, hydroxyl, amino, alkylamino, dialkylamino, acylamino, thiol, alkylthio, cyano, carbonyl, carboxyl, alkoxycarbonyl, nitro, acyl, acyloxy, alkyl, haloalkyl, heteroalkyl, alkoxy, alkenyl, alkynyl, sulfinyl, sulfonyl, thiocarbonyl, carbamoyl, cycloalkyl, heterocyclyl, aryl or heteroaryl;
each of R9, R10, R11 and R12 independently are hydrogen, halogen, hydroxyl, amino, alkylamino, dialkylamino, acylamino, thiol, alkylthio, cyano, carbonyl, carboxyl, alkoxycarbonyl, nitro, acyl, acyloxy, alkyl, haloalkyl, heteroalkyl, alkoxy, alkenyl, alkynyl, sulfinyl, sulfonyl, thiocarbonyl, carbamoyl, cycloalkyl, heterocyclyl, aryl or heteroaryl, and each of which can be optionally substituted; or
at least a vicinal pair formed from selecting two of R9, R10, R11 or R12 and the carbons to which they are attached form an optionally substituted 5- or 6-member cycloalkyl or an optionally substituted 5- or 6-member heterocyclyl, and the remaining R9, R10, R11 or R12 independently are hydrogen, halogen, hydroxyl, amino, alkylamino, dialkylamino, acylamino, thiol, alkylthio, cyano, carbonyl, carboxyl, alkoxycarbonyl, nitro, acyl, acyloxy, alkyl, haloalkyl, heteroalkyl, alkoxy, alkenyl, alkynyl, sulfinyl, sulfonyl, thiocarbonyl, carbamoyl, cycloalkyl, heterocyclyl, aryl or heteroaryl, and each of which can be optionally substituted; and
wherein any alkoxy, alkylamino, dialkylamino, acylamino, alkylthio, carbonyl, carboxyl, alkoxycarbonyl, acyl, acyloxy, alkyl, haloalkyl, heteroalkyl, alkenyl, alkynyl, sulfinyl, sulfonyl, thiocarbonyl, carbamoyl, cycloalkyl, heterocyclyl, aryl and heteroaryl can be optionally substituted with 1, 2, 3 or 4 substituents independently selected from the group consisting of halogen, hydroxy, carboxyl, oxo, nitro, haloalkyl, alkyl, alkenyl, alkynyl, alkaryl, aryl, heteroaryl, cycloalkyl, heterocyclyl, aralkyl, alkoxy, aryloxy, amino, acylamino, alkylcarbanoyl, arylcarbanoyl, aminoalkyl, alkoxycarbonyl, carboxy, hydroxyalkyl, alkanesulfonyl, arenesulfonyl, alkanesulfonamido, arenesulfonamido, aralkylsulfonamido, alkylcarbonyl, acyloxy, cyano and ureido,
and where any heterocyclyl is a nonaromatic 3-8 membered monocyclic, 8-12 membered bicyclic, or 11-14 membered tricyclic ring system having 1-3 heteroatoms if monocyclic, 1-6 heteroatoms if bicyclic, or 1-9 heteroatoms if tricyclic, said heteroatoms selected independently from O, N, and S.
|