| CPC C07D 403/14 (2013.01) [A61K 9/1605 (2013.01); A61K 45/06 (2013.01); A61P 35/00 (2018.01)] | 4 Claims |
|
1. A method of treating synovial sarcoma in a subject in need thereof, the method comprising administering to the subject an effective amount of a compound having the structure of Formula I:
A-L-B Formula I
wherein
L is a linker;
B is a degradation moiety, which has the structure of Formula A-1:
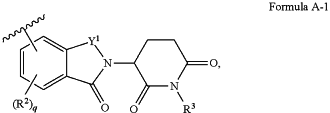 wherein
Y1 is
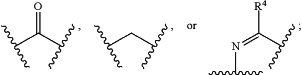 each of R3 and R4 is, independently, H, optionally substituted C1-C6 alkyl, or optionally substituted C1-C6 heteroalkyl;
q is 0, 1, 2, 3, or 4; and
each R2 is, independently, halogen, optionally substituted C1-C6 alkyl, optionally substituted C1-C6 heteroalkyl, optionally substituted C3-C10 carbocyclyl, optionally substituted C2-C9 heterocyclyl, optionally substituted C6-C10 aryl, optionally substituted C2-C9 heteroaryl, optionally substituted C2-C6 alkenyl, optionally substituted C2-C6 heteroalkenyl, hydroxyl, —SH, or optionally substituted amino;
A has the structure of Formula E-a:
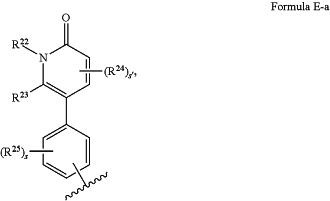 where
R22 is H, optionally substituted C1-C6 alkyl, or optionally substituted C1-C6 heteroalkyl;
R23 is H, halogen, optionally substituted C1-C6 alkyl, or optionally substituted C6-C10 aryl;
s′ is 0, 1, or 2;
each R24 is, independently, halogen, optionally substituted C1-C6 alkyl, optionally substituted C1-C6 heteroalkyl, optionally substituted C3-C10 carbocyclyl, optionally substituted C2-C9 heterocyclyl, optionally substituted C6-C10 aryl, optionally substituted C2-C9 heteroaryl, optionally substituted C2-C6 alkenyl, optionally substituted C2-C6 heteroalkenyl, hydroxyl, —SH, or optionally substituted amino, or two R24 combine with the carbon atoms to which they are attached to form an optionally substituted C6-C10 aryl or optionally substituted C2-C9 heteroaryl;
s is 0, 1, 2, 3, or 4; and
each R25 is, independently, halogen, optionally substituted C1-C6 alkyl, optionally substituted C1-C6 heteroalkyl, optionally substituted C3-C10 carbocyclyl, optionally substituted C2-C9 heterocyclyl, optionally substituted C6-C10 aryl, optionally substituted C2-C9 heteroaryl, optionally substituted C2-C6 alkenyl, optionally substituted C2-C6 heteroalkenyl, hydroxyl, —SH, or optionally substituted amino, or a pharmaceutically acceptable salt thereof.
|