| CPC C07C 41/18 (2013.01) [B01J 31/181 (2013.01); B01J 31/1815 (2013.01); B01J 31/2295 (2013.01); C07C 1/20 (2013.01); B01J 2531/821 (2013.01); C07C 2601/16 (2017.05)] | 10 Claims |
|
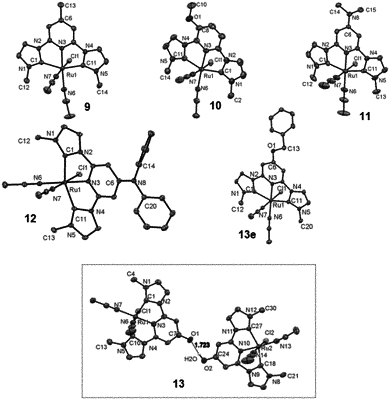
1. A method comprising:
selectively deoxygenating at least one oxygenated aromatic compound in the presence of a hydrogen gas and a catalyst system to form a reaction product, wherein the catalyst system comprises a complex of formula (I):
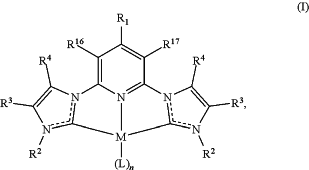 wherein
R1 is hydrogen, OH, halogen, amine, C1-C10 alkyl, C1-C10 alkoxy, C2-C10 alkenyl, C2-C10 alkynyl, C6-C14 aryl, C1-C13 heteroaryl, C6-C14 aryloxy, C3-C10 cycloalkyl, or C3-C10 cycloalkenyl, wherein R1 is optionally substituted with C1-C10 alkyl, C1-C10 alkoxy, C2-C10 alkenyl, C2-C10 alkynyl, C6-C14 aryl, C1-C13 heteroaryl, aldehyde, amino, carboxylic acid, ester, ether, halide, hydroxy, ketone, nitro, cyano, silyl, sulfo-oxo, sulfonyl, sulfone, sulfoxide, thiol, phosphonyl;
each R2 is, independent of the other, C1-C10 alkyl, C2-C10 alkenyl, C2-C10 alkynyl, C6-C14 aryl, or C1-C13 heteroaryl, wherein R2 is optionally substituted with C1-C10 alkyl, C1-C10 alkoxy, C2-C10 alkenyl, C2-C10 alkynyl, C6-C14 aryl, C1-C13 heteroaryl, aldehyde, amino, carboxylic acid, ester, ether, halide, hydroxy, ketone, nitro, cyano, silyl, sulfo-oxo, sulfonyl, sulfone, sulfoxide, or thiol;
each R3 and R4 are, independent of the other, hydrogen, C1-C10 alkyl, C2-C10 alkenyl, C2-C10 alkynyl, C6-C14 aryl, or C1-C13 heteroaryl, wherein R3 and R4 are optionally substituted with C1-C10 alkyl, C1-C10 alkoxy, C2-C10 alkenyl, C2-C10 alkynyl, C6-C14 aryl, C1-C13 heteroaryl, aldehyde, amino, carboxylic acid, ester, ether, halide, hydroxy, ketone, nitro, cyano, silyl, sulfo-oxo, sulfonyl, sulfone, sulfoxide, or thiol, or R3 and R4 combine together with the atoms to which they are attached to form a cycloalkene ring, heteroaromatic ring, or aromatic ring;
each R16 and R17 are, independent of the other, hydrogen, OH, halogen, amine, C1-C10 alkyl, C1-C10 alkoxy, C2-C10 alkenyl, C2-C10 alkynyl, C6-C14 aryl, C1-C13 heteroaryl, C6-C14 aryloxy, C3-C10 cycloalkyl, or C3-C10 cycloalkenyl, wherein each R16 and R15, independent of the other, is optionally substituted with C1-C10 alkyl, C1-C10 alkoxy, C2-C10 alkenyl, C2-C10 alkynyl, C6-C14 aryl, C1-C13 heteroaryl, aldehyde, amino, carboxylic acid, ester, ether, halide, hydroxy, ketone, nitro, cyano, silyl, sulfo-oxo, sulfonyl, sulfone, sulfoxide, thiol, or phosphonyl;
M is Ru or Ir;
each L is independently selected from C1, Br, CH3CN, DMF, H2O, bipyridine, phenylpyridine, CO2, and a CNC-pincer ligand; and
n is 1, 2, or 3;
wherein the oxygenated aromatic compound has the formula:
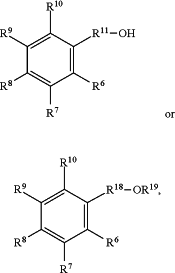 wherein
R6 is independently selected from hydrogen, substituted or unsubstituted C1-C6-alkyl, substituted or unsubstituted C3-C10 cycloalkyl and heteroalkyl, C2-C10 alkenyl, and Ar′;
R7 is independently selected from hydrogen, substituted or unsubstituted C1-C6-alkyl, substituted or unsubstituted C3-C10 cycloalkyl and heteroalkyl, C2-C10 alkenyl, and Ar′;
R8 is independently selected from hydrogen, substituted or unsubstituted C1-C6-alkyl, substituted or unsubstituted C3-C10 cycloalkyl and heteroalkyl, C2-C10 alkenyl, and Ar′;
R9 is independently selected from hydrogen, substituted or unsubstituted C1-C6-alkyl, substituted or unsubstituted C3-C10 cycloalkyl and heteroalkyl, C2-C10 alkenyl, and Ar′;
R10 is independently selected from hydrogen, substituted or unsubstituted C1-C6-alkyl; substituted or unsubstituted C3-C10 cycloalkyl and heteroalkyl, C2-C10 alkenyl, and Ar′;
wherein R11 is a bond, substituted or unsubstituted C1-C6 alkylene, C2-C10 alkenylene, or Ar″;
R18 is independently selected from substituted or unsubstituted C1-C6-alkylene, C2-C10 alkenylene, and Ar″;
R19 is independently selected from substituted or unsubstituted C1-C6-alkyl, substituted or unsubstituted C3-C10 cycloalkyl and heteroalkyl, C2-C10 alkenyl, and Ar′;
Ar′ is a C6-C14 aryl or heteroaryl group optionally substituted with 1, 2, or 3 substituents;
Ar″ is a C6-C14 arylene or heteroarylene group optionally substituted with 1, 2, or 3 substituents;
wherein Ar′ and Ar″ are the same or different, and
wherein the reaction product has the formula:
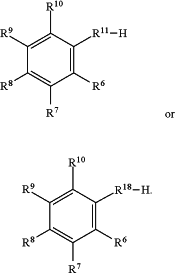 |