| CPC A61K 31/44 (2013.01) [A61K 9/0053 (2013.01); A61K 31/167 (2013.01); A61K 31/416 (2013.01); A61K 31/444 (2013.01); A61K 31/496 (2013.01); A61K 39/3955 (2013.01); A61P 3/06 (2018.01); A61P 3/10 (2018.01); A61P 9/10 (2018.01); A61P 11/00 (2018.01); A61P 35/00 (2018.01); C07C 233/65 (2013.01); C07D 213/73 (2013.01); C07D 231/56 (2013.01); C07D 401/12 (2013.01); C07D 401/14 (2013.01)] | 42 Claims |
|
1. A method of inhibiting dihydroceramide desaturase (Des) activity in a biological sample comprising contacting the biological sample with a compound of Formula I:
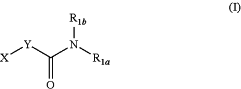 or a salt thereof, wherein:
R1a and R1b are independently selected from hydrogen, cycloalkylalkyl, aryl, and heteroaryl, and is optionally substituted with 1, 2, or 3 R2 groups, or
R1a and R1b together with the intervening atoms, form a 5-7 membered heterocyclic ring, and is optionally substituted with 1, 2, or 3 R2 groups;
at least one of R1a and R1b is not hydrogen;
X is selected from ethenyl, alkyl, aryl, biaryl, (aryl)cycloalkyl, (aryl)heterocycloalkyl, (aryl)heteroaryl, cycloalkyl, (cycloalkyl)aryl, (cycloalkyl)cycloalkyl, (cycloalkyl)heterocycloalkyl, (cycloalkyl)heteroaryl, heterocycloalkyl, (hetercycloalkyl)aryl, (heterocycloalkyl)cycloalkyl, (heterocycloalkyl)heteroaryl, (heterocycloalkyl)heterocycloalkyl, heteroaryl, (heteroaryl)aryl, (heteroaryl)cycloalkyl, (heteroaryl)heterocycloalkyl, and (heteroaryl)heteroaryl, any of which is optionally substituted with 1, 2, 3, or 4 R3 groups;
Y is selected from —CHR4—,—CHR4CHR4—, and —CR4=CR4;
each R2 is independently selected from alkyl, alkoxy, amino, cycloalkyl, heterocycloalkyl, (heterocycloalkyl)alkyl, halogen, hydroxy, S-sulfonamido, and oxo, and is optionally substituted with 1, 2, or 3 R5
n is selected from 1, 2, 3, 4, and 5;
each R3 is independently selected from alkyl, alkoxy, cyano, haloalkyl, hydroxy, halogen, and oxo; and
each R4 is independently selected from hydrogen, alkyl, alkylamino, halo, and hydroxyl; and
each R5 is independently selected from hydroxy and alkoxy.
|