| CPC C01B 32/184 (2017.08) [B82Y 30/00 (2013.01); B82Y 40/00 (2013.01); C01B 2204/02 (2013.01); C01B 2204/04 (2013.01); C01B 2204/32 (2013.01); Y10S 977/734 (2013.01); Y10S 977/842 (2013.01); Y10S 977/845 (2013.01)] | 17 Claims |
|
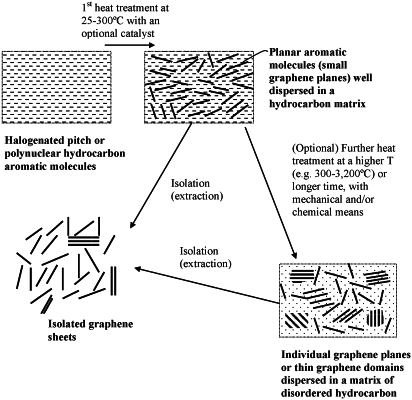
1. A method of producing isolated graphene sheets, said method comprising:
a) providing a mass of halogenated aromatic molecules in a solid, or semi-solid state wherein said halogenated aromatic molecules are selected from halogenated variants of petroleum heavy oil or pitch, coal tar pitch, a polynuclear hydrocarbon, and combinations thereof, wherein said halogenated variants of polynuclear hydrocarbon are selected from halogenated variants of naphthalene, phenanthrene, chrysene, triphenylene, corannulene, benzo-perylene, coronene, ovalene, benzo-fluorene, a derivative thereof having a substituent on a ring structure thereof, a chemical derivative thereof, and combinations thereof;
b) heat treating said mass of halogenated aromatic molecules at a first temperature selected from 25° C. to 3,000° C. so that said halogenated aromatic molecules are merged or fused into larger aromatic molecules and optionally heat-treating said larger molecules at a second temperature, higher than the first temperature, selected from 300° C. to 3,200° C., to form graphene domains dispersed in a disordered matrix of carbon or hydrocarbon molecules, wherein said graphene domains are each composed of from 1 to 30 planes of hexagonal carbon atoms or fused aromatic rings having a length or width from 5 nm to 35 μm and, in the situations wherein there are 2-30 planes in a graphene domain, having an inter-graphene space between two planes of hexagonal carbon atoms or fused aromatic rings no less than 0.34 nm;
c) separating and isolating said planes of hexagonal carbon atoms or fused aromatic rings to recover graphene sheets from said disordered matrix; and
d) conducting a chemical means to treat said halogenated aromatic molecules during or after said step (b), wherein said chemical means comprises adding a functionalizing agent into said mass of halogenated aromatic molecules and said halogenated aromatic molecules are chemically functionalized by said agent, wherein said functionalizing agent contains a functional group selected from the group consisting of amidoamines, polyamides, aliphatic amines, modified aliphatic amines, cycloaliphatic amines, aromatic amines, anhydrides, ketimines, diethylenetriamine (DETA), triethylene-tetramine (TETA), tetraethylene-pentamine (TEPA), polyethylene polyamine, polyamine epoxy adduct, phenolic hardener, non-brominated curing agent, non-amine curatives, an acrylonitrile chain, polyfurfuryl alcohol, phenolic resin, and combinations thereof; and/or said functionalizing agent contains a functional group selected from OY, NHY, O═C—OY, P═C—NR′Y, O═C—SY, O═C—Y, —CR′1-OY, N′Y or C′Y, and Y is a functional group of a protein, a peptide, an amino acid, an enzyme, an antibody, a nucleotide, an oligonucleotide, an antigen, or an enzyme substrate, enzyme inhibitor or the transition state analog of an enzyme substrate or is selected from R′—OH, R′—NR′2, R′SH, R′CHO, R′CN, R′X, R′N+(R′)3X−, R′SiR′3, R′Si(—OR′—)yR′3-y, R′Si(—O—SiR′2—)OR′, R′—R″, R′—N—CO, (C2H4O—)wH, (—C3H6O—)wH, (—C2H4O)w—R′, (C3H6O)w—R′, R′, and w is an integer greater than one and less than 200;
wherein said graphene sheet production and said chemical functionalization are accomplished concurrently.
|