| CPC A61K 9/0024 (2013.01) [A61K 31/567 (2013.01)] | 17 Claims |
|
1. A non-injectable biodegradable contraceptive implant for implantation in tissue in a female subject comprising
a contraceptive agent free biodegradable core having a coating thereon, the coating being formed of a biocompatible polyester copolymer,
wherein the biodegradable polyester copolymer is poly(ω-pentadecalactone-co-p-dioxanone) having a general structure according to Formula (I)
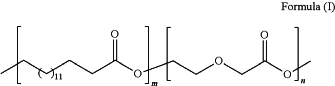 wherein n and m are independently integer values of less than or equal to about 1500, and
having in the coating on the core, but not in the core, an effective amount of a contraceptive agent being released with zero order kinetics to prevent pregnancy in the female subject over a period of twelve to twenty-four months, and
an outer biodegradable poly(ω-pentadecalactone-co-p-dioxanone) coating on the coating on the core, wherein the outer coating does not contain contraceptive agent.
|