| CPC G01N 27/02 (2013.01) [G01N 33/5438 (2013.01); G01N 33/6803 (2013.01); G01N 27/028 (2013.01)] | 17 Claims |
|
1. A method for identifying the presence of a biomarker in a biological sample obtained from a mammalian subject who may be at risk of developing, a disease, disorder or condition, comprising:
functionalizing a solid support to increase its affinity for a biomarker associated with a disease, disorder or condition;
mixing, the functionalized solid support and a biological sample to form a test sample;
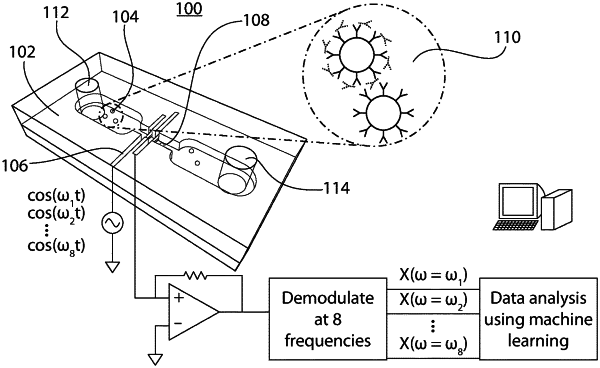
providing a platform comprising at least one channel, a first electrode for sending a signal and a second electrode for receiving the signal;
inserting the test sample into a channel of the platform;
using multi-channel impedance responses from frequencies ranging from 600 kHz to 25 MHz to calculate an impedance of the test sample based on the signal sent by the first electrode and received by the second electrode when the test sample is located adjacent to the first and second electrodes, wherein the impedance value is used to distinguish between a solid support bound to a biomarker and a solid support without a biomarker bound thereto; and
identifying the presence of the biomarker associated with a disease, disorder or condition based on the calculated impedance of the test sample;
wherein the disease, disorder or condition is a disease, disorder or condition of the oral cavity.
|