| CPC A61K 47/65 (2017.08) [A61K 31/155 (2013.01); A61K 31/519 (2013.01); A61K 47/64 (2017.08); A61P 31/14 (2018.01)] | 20 Claims |
|
1. An antiviral composition comprising a plurality of functionalized twin base linker (TBL) molecules having the general structure
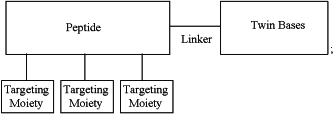 wherein the twin bases comprise a structure according to Formula 1
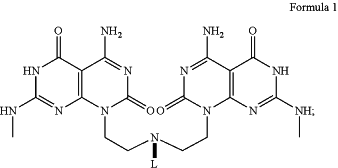 wherein L is the linker and comprises carbon, nitrogen, and/or oxygen atoms and has a chain length from about 4 to about 20 atoms;
wherein the peptide moiety serves as a backbone and contains from about 2 to about 20 L- and/or D-amino acids; and
wherein one or more targeting moieties are covalently linked to the peptide, and wherein each of the one or more targeting moieties consists of from 3 to 12 amino acids and is capable of specifically binding a surface-accessible protein of a virus, thereby deactivating the virus.
|