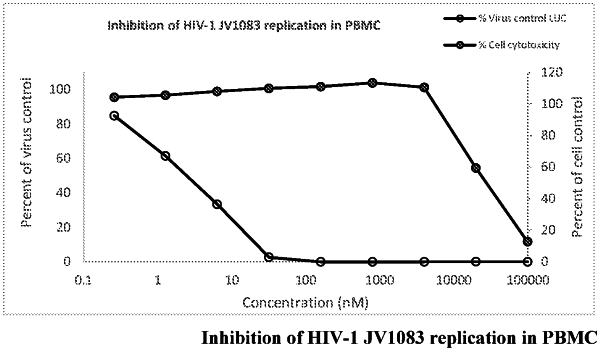
|
1. A pharmaceutical composition comprising ((((((R)-1-(6-amino-9H-purin-9-yl)propan-2-yl)oxy)methyl)(phenoxy)phosphoryl)oxy)methyl pivalate in the form of a fumarate salt or a pharmaceutically acceptable derivative thereof in an amount from about 2% to about 80% of the composition, one or more pharmaceutically acceptable excipients, and a therapeutic agent selected from the group consisting of: emtricitabine, efavirenz, nevirapine, darunavir, atazanavir, cobicistat, dolutegravir, elvitegravir, raltegravir, and any combination thereof.
|