| CPC H10K 50/11 (2023.02) [C07F 15/0086 (2013.01); C09K 11/06 (2013.01); H10K 50/131 (2023.02); H10K 50/155 (2023.02); H10K 50/156 (2023.02); H10K 85/346 (2023.02); C09K 2211/1044 (2013.01); C09K 2211/185 (2013.01); H10K 2101/10 (2023.02); H10K 2101/30 (2023.02); H10K 2101/40 (2023.02); H10K 2101/90 (2023.02)] | 16 Claims |
|
1. An organic light-emitting device comprising:
a first electrode;
a second electrode facing the first electrode; and
an organic layer disposed between the first electrode and the second electrode,
wherein
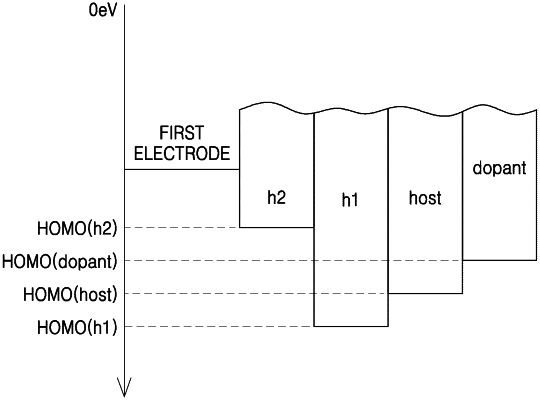
the organic layer comprises an emission layer, and an intermediate layer disposed between the first electrode and the emission layer,
the intermediate layer directly contacts the emission layer and comprises a first hole transport material,
the emission layer comprises a host and a dopant,
the dopant is an organometallic compound, provided that the dopant does not comprise iridium, and
the organic light-emitting device satisfies conditions of HOMO(h1)−HOMO(host)<0 electron volts and HOMO(host)<HOMO(dopant),
wherein HOMO(h1) indicates the highest occupied molecular orbital (HOMO) energy level (expressed in electron volts) of the first hole transport material,
i) when the host consists of a single host, the HOMO(host) indicates a HOMO energy level (expressed in electron volts) of the single host and ii) when the host is a mixture of two or more different hosts, the HOMO(host) indicates the highest energy level among HOMO energy levels (expressed in electron volts) of the two or more different hosts,
wherein HOMO(dopant) is a HOMO energy level (expressed in electron volts) of the dopant included in the emission layer, and
HOMO(h1), HOMO(host), and HOMO(dopant) are measured by using a photoelectron spectrometer in an ambient atmosphere,
wherein the dopant is represented by Formula 1A-1:
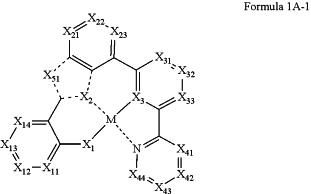 wherein, in Formula 1A-1,
M is platinum,
X1 is O or S, and a bond between X1 and M is a covalent bond,
X2 is N,
X3 is C,
a bond between X2 and M is a coordinate bond,
a bond between X3 and M is a covalent bond,
X11 is N or C-[(L11)b11-(R11)c11], X12 is N or C-[(L12)b12-(R12)c12], X13 is N or C-[(L13)b13-(R13)c13], and X14 is N or C-[(L14)b14-(R14)c14],
X21 is N or C-[(L21)b21-(R21)c21], X22 is N or C-[(L22)b22-(R22)c22], and X23 is N or C-[(L23)b23-(R23)c23],
X31 is N or C-[(L31)b31-(R31)c31], X32 is N or C-[(L32)b32-(R32)c32], and X33 is N or C-[(L33)b33-(R33)c33],
X41 is N or C-[(L41)b41-(R41)c41], X42 is N or C-[(L42)b42-(R42)c42], X43 is N or C-[(L43)b43-(R43)c43], and X44 is N or C-[(L44)b44-(R44)c44],
X51 is N-[(L7)b7-(R7)c7], C(R7)(R8), Si(R7)(R8), Ge(R7)(R8), C(═O), N, C(R7), Si(R7), or Ge(R7),
with the proviso that when X51 is N-[(L7)b7-(R7)c7], L7 is a phenyl group, and b7 is 1, then c7 is two or more, at least one R7 comprises an alkyl group, and one R7 comprises an aryl group,
R7 and R8 are optionally linked via a first linking group to form a substituted or unsubstituted C5-C30 carbocyclic group or a substituted or unsubstituted C1-C30 heterocyclic group,
L7, L11 to L14, L21 to L23, L31 to L33, and L41 to L44 are each independently a substituted or unsubstituted C5-C30 carbocyclic group or a substituted or unsubstituted C1-C30 heterocyclic group,
7 is an integer from 1 to 5,
b11 to b14, b21 to b23, b31 to b33, and b41 to b44 are each independently an integer from 0 to 5,
c7, c11 to c14, c21 to c23, c31 to c33, and c41 to c44 are each independently an integer from 1 to 5,
R7, R8, R11 to R14, R21 to R23, R31 to R33, and R41 to R44 are each independently hydrogen, deuterium, —F, —Cl, —Br, —I, —SF5, a hydroxyl group, a cyano group, a nitro group, an amidino group, a hydrazine group, a hydrazone group, a carboxylic acid group or a salt thereof, a sulfonic acid group or a salt thereof, a phosphoric acid group or a salt thereof, a substituted or unsubstituted C1-C60 alkyl group, a substituted or unsubstituted C2-C60 alkenyl group, a substituted or unsubstituted C2-C60 alkynyl group, a substituted or unsubstituted C1-C60 alkoxy group, a substituted or unsubstituted C3-C10 cycloalkyl group, a substituted or unsubstituted C1-C10 heterocycloalkyl group, a substituted or unsubstituted C3-C10 cycloalkenyl group, a substituted or unsubstituted C1-C10 heterocycloalkenyl group, a substituted or unsubstituted C6-C60 aryl group, a substituted or unsubstituted C6-C60 aryloxy group, a substituted or unsubstituted C6-C60 arylthio group, a substituted or unsubstituted C7-C60 arylalkyl group, a substituted or unsubstituted C1-C60 heteroaryl group, a substituted or unsubstituted C1-C60 heteroaryloxy group, a substituted or unsubstituted C1-C60 heteroarylthio group, a substituted or unsubstituted C2-C60 heteroarylalkyl group, a substituted or unsubstituted monovalent non-aromatic condensed polycyclic group, a substituted or unsubstituted monovalent non-aromatic condensed heteropolycyclic group, —N(Q1)(Q2), —Si(Q3)(Q4)(Q5), —B(Q6)(Q7), and —P(═O)(Q8)(Q9), provided that R7 is not hydrogen,
two or more of R11 to R14 are optionally linked to form a substituted or unsubstituted C5-C30 carbocyclic group or a substituted or unsubstituted C1-C30 heterocyclic group,
two or more of R21 to R23 are optionally linked to form a substituted or unsubstituted C5-C30 carbocyclic group or a substituted or unsubstituted C1-C30 heterocyclic group,
two or more of R31 to R33 are optionally linked to form a substituted or unsubstituted C5-C30 carbocyclic group or a substituted or unsubstituted C1-C30 heterocyclic group,
two or more of R41 to R44 are optionally linked to form a substituted or unsubstituted C5-C30carbocyclic group or a substituted or unsubstituted C1-C30 heterocyclic group, and
Q1 to Q9 are each independently selected from hydrogen, deuterium, —F, —Cl, —Br, —I, a hydroxyl group, a cyano group, a nitro group, an amidino group, a hydrazine group, a hydrazone group, a carboxylic acid group or a salt thereof, a sulfonic acid group or a salt thereof, a phosphoric acid group or a salt thereof, a C1-C60 alkyl group, a C1-C60 alkyl group substituted with at least one selected from deuterium, a C1-C60 alkyl group, and a C6-C60 aryl group, a C2-C60 alkenyl group, a C2-C60 alkynyl group, a C1-C60 alkoxy group, a C3-C10 cycloalkyl group, a C1-C10 heterocycloalkyl group, a C3-C10 cycloalkenyl group, a C1-C10 heterocycloalkenyl group, a C6-C60 aryl group, a C6-C60 aryl group substituted with at least one selected from deuterium, a C1-C60 alkyl group, and a C6-C60 aryl group, a C6-C60 aryloxy group, a C6-C60 arylthio group, a C7-C60 arylalkyl group, a C1-C60 heteroaryl group, a C1-C60 heteroaryloxy group, a C1-C60 heteroarylthio group, a C2-C60 heteroarylalkyl group, a monovalent non-aromatic condensed polycyclic group, and a monovalent non-aromatic condensed heteropolycyclic group.
|