| CPC H01M 4/38 (2013.01) [H01M 4/362 (2013.01); H01M 4/625 (2013.01); H01M 10/0525 (2013.01); H01M 2004/021 (2013.01); H01M 2004/028 (2013.01); H01M 2220/20 (2013.01); H01M 2300/0037 (2013.01)] | 11 Claims |
|
1. A lithium secondary battery comprising:
a positive electrode comprising a positive electrode active material layer;
a negative electrode;
a separator; and
an electrolyte liquid,
wherein the lithium secondary battery has an ED factor value represented by the following Mathematical Formula 1 of 850 or greater and 10,000 or less:
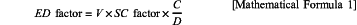 in Mathematical Formula 1, V is a discharge nominal voltage (V) for Li/Li+, D is a density (g/cm3) of the electrolyte liquid, C is a discharge capacity (mAh/g) of the lithium secondary battery when discharging at a 0.1 C rate, and the SC factor is represented by the following Mathematical Formula 2, the SC factor being 0.45 or greater and 4.5 or less:
 in Mathematical Formula 2, P is porosity (%) of the positive electrode active material layer, L is a mass of sulfur per unit area (mg/cm2) of the positive electrode active material layer, and α is 10, wherein the SC factor defines the relationship between α, P, and L;
and
further wherein the positive electrode comprises a sulfur-carbon composite comprising a carbon material satisfying a condition of an AC factor represented by the following Mathematical Formula 3 being 100 or greater and 1,000 or less:
AC factor=0.1×specific area(m2/g)+2×conductivity(S/cm@2000 kgf) [Mathematical Formula 3]
in Mathematical Formula 3, specific area is a specific surface area of the carbon material, and conductivity is electrical conductivity obtained by converting a powder resistance value measured while applying a pressure of 2000 kgf to the carbon material to conductivity,
wherein the electrolyte liquid comprises a solvent and a lithium salt, and the solvent comprises a first solvent in which a DV2 factor value represented by the following Mathematical Formula 4 is 0.1 or greater and 1.75 or less and a second solvent that is a fluorinated ether-based solvent:
 in Mathematical Formula 4,
DV is a dipole moment per unit volume (D·mol/L);
μ is viscosity of the solvent (cP, 25° C.); and
γ is 100,
wherein the DV2 factor defines the relationship between μ, γ, and DV, and
wherein the positive electrode comprises a dot-type carbon material.
|