| CPC C07K 16/44 (2013.01) [A61K 9/0019 (2013.01); A61K 31/4192 (2013.01); A61K 45/06 (2013.01); A61K 47/54 (2017.08); A61K 47/549 (2017.08); A61K 47/55 (2017.08); A61K 47/646 (2017.08); A61K 47/68 (2017.08); A61K 47/6803 (2017.08); A61K 47/6869 (2017.08); A61K 47/6873 (2017.08); A61K 47/6891 (2017.08); C07D 249/04 (2013.01); C07K 16/3069 (2013.01); C07K 2317/31 (2013.01)] | 30 Claims |
|
1. A composition in pharmaceutical dosage form in combination with a pharmaceutically acceptable carrier, additive or excipient, comprising an anticancer effective amount of a compound according to the chemical structure:
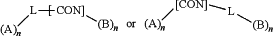 Wherein each n is independently 1 or 2;
A is an antibody binding moiety according to the chemical structure:
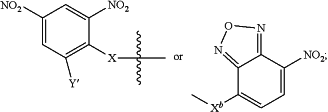 Where Y′ is H or NO2;
X is O, CH2, NR1, S(O), S(O)2, —S(O)2O, —OS(O)2, or OS(O)2O;
R1 is H, a C1-C3 alkyl group, or a —C(O)(C1-C3) group;
Xb is a bond, O, CH2, NR1 or S;
B is a cell binding moiety according to the chemical formula:
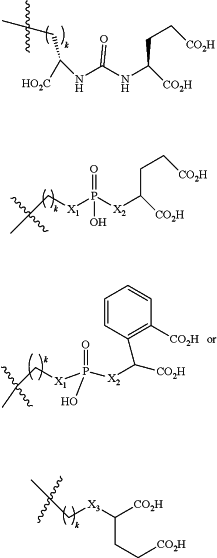 Wherein X1 and X2 are each independently CH2, O, NH or S;
X3 is O, CH2, NR1, S(O), S(O)2, —S(O)2O, —OS(O)2, or OS(O)2O;
R1 is H, a C1-C3 alkyl group, or a —C(O)(C1-C3) group;
k is an integer from 1 to 10;
L is a linker according to the chemical formula:
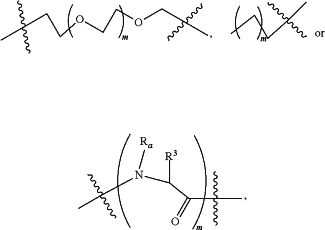 Or L is a polyethylene glycol, polypropylene glycol or polypropylene-co-polyethylene glycol linker having between 1 and 20 glycol units;
Wherein Ra is H, C1-C3 alkyl or alkanol or together with Ra forms a proline side chain with R3;
R3 forms a proline side chain with Ra or R3 is a side chain derived from an amino acid selected from the group consisting of alanine, arginine, asparagine, aspartic acid, cysteine, glutamine, glutamic acid, glycine, histidine, isoleucine, leucine, lysine, methionine, phenylalanine, serine, threonine, tryptophan and valine; and
Each m is independently an integer from 1 to 12; or
L is a linker according to the chemical formula:
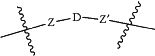 Where Z and Z′ are each independently a bond, —(CH2)i-O, —(CH2)i-S, —(CH2)i-N-R,
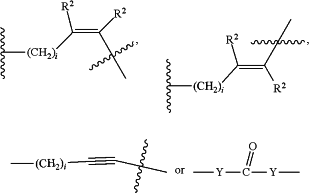 wherein said —(CH2)i group, if present in Z or Z′, is bonded to[CON], antibody binding terminus (ABT) or cell binding terminus (CBT);
Each R is independently H, or a C1-C3 alkyl or alkanol group;
Each R2 is independently H or a C1-C3 alkyl group;
Each Y is independently a bond, O, S or N-R;
Each i is independently an integer from 0 to 15;
D is
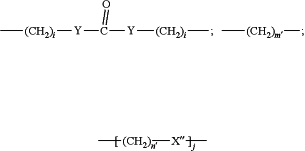 or a bond,
with the proviso that Z, Z′ and D are not each simultaneously bonds;
j is an integer from 1 to 100;
m′ is an integer from 1 to 100;
n′ is an integer from 1 to 100; and
X″ is O, S or N-R,
R is as defined above; and
[CON] is a bond or a moiety according to the chemical structure:
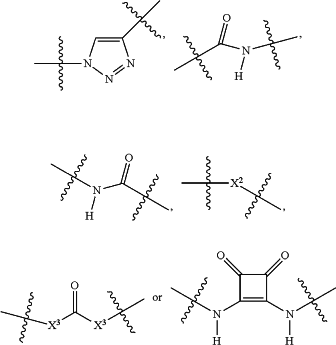 Where X2 is O, S, NR4, S(O), S(O)2, —S(O)2O, —OS(O)2, or OS(O)2O;
X3 is NR4, O or S; and R4 is H, a C1-C3 alkyl or alkanol group, or a —C(O)(C1-C3) group; or
a pharmaceutically acceptable salt thereof.
|