| CPC C07D 519/00 (2013.01) [A61P 35/00 (2018.01); A61P 35/02 (2018.01)] | 14 Claims |
|
1. A compound of Formula (I)
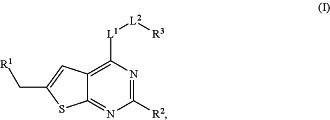 or a tautomer or a stereoisomeric form thereof, wherein
R1 is selected from the group consisting of CH3, CH2F, CHF2, and CF3;
R2 is selected from the group consisting of hydrogen and CH3;
L1 represents a 7- to 10-membered saturated spiroheterobicyclic system containing one or two N-atoms provided that it is N-linked to the thienopyrimidinyl heterocycle; and
-L2-R3 is selected from (d), (e), (g) or (h), wherein
(d) L2 is O and R3 is selected from the group consisting of C3-6alkyl optionally substituted with one, two or three fluoro substituents; Ar; Het1; Het2; a 7- to 10-membered saturated spirocarbobicyclic system; —CH2—Ar; —CH2—Het1; —CH2—Het2; and —CH2-(a 7- to 10-membered saturated spirocarbobicyclic system); when L2 is linked to a carbon atom of L1; or
(e) -L2-R3 is —O—CHR5—R3 when L2 is linked to a carbon atom of L1, wherein
R5 is selected from the group consisting of —C(═O)NR13aR13b; C1-4alkyl optionally substituted with a substituent selected from the group consisting of fluoro, —OR14, and —NR15aR15b; and C-linked 4- to 7-membered non-aromatic heterocyclyl containing at least one nitrogen, oxygen or sulfur atom; wherein
R13a, R13b, R14, R15a and R15b are each independently selected from the group consisting of hydrogen; C1-4alkyl optionally substituted with a substituent selected from the group consisting of fluoro and —C(═O)NR16aR16b; and
C2-4alkyl substituted with a substituent selected from the group consisting of —OR17 and —NR16aR16b; wherein
R16a, R16b and R17 are each independently selected from the group consisting of hydrogen; C1-4alkyl; and C-linked 4- to 7-membered non-aromatic heterocyclyl containing at least one nitrogen, oxygen or sulfur atom; and
R3 is selected from the group consisting of hydrogen; C1-4alkyl optionally substituted with one, two, or three fluoro substituents; —CN; Ar, Het1; Het2; and a 7- to 10-membered saturated spirocarbobicyclic system; or
(g) -L2-R3 is
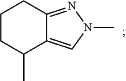 and wherein
Ar is phenyl or naphthyl, each of which may be optionally substituted with one, two, or three substituents each independently selected from the group consisting of halo, —CN, —OR24, —NR25aR25b, and
C1-4alkyl optionally substituted with a substituent selected from the group consisting of fluoro, —CN, —OR26, —NR27aR27b, and —C(═O)NR27aR27b;
Het1 is a monocyclic heteroaryl selected from the group consisting of pyridyl, 4-, 5- or 6-pyrimidinyl, pyrazinyl, pyridazinyl, furanyl, thienyl, pyrrolyl, pyrazolyl, imidazolyl, 4- or 5-thiazolyl, isothiazolyl, thiadiazolyl, and isoxazolyl; or a bicyclic heteroaryl selected from the group consisting of imidazothiazolyl, imidazoimidazolyl, benzofuranyl, benzothiophenyl, benzimidazolyl, benzoxazolyl, isobenzoxazolyl, benzisoxazolyl, benzothiazolyl, benzisothiazolyl, isobenzofuranyl, indolyl, isoindolyl, indolizinyl, indolinyl, isoindolinyl, indazolyl, pyrazolopyridinyl, pyrazolopyrimidinyl, imidazopyridinyl, imidazopyrazinyl, imidazopyridazinyl; each of which may be optionally substituted with one, two, or three substituents each independently selected from the group consisting of halo, —CN, —OR24, —NR25aR25b and C1-4alkyl optionally substituted with a substituent selected from the group consisting of fluoro, —CN, —OR26, —NR27aR27b, and —C(═O)NR27aR27b; and
Het2 is a non-aromatic heterocyclyl optionally substituted with one, two, or three substituents each independently selected from the group consisting of halo, —CN, —OR24, —NR25aR25b, and C1-4alkyl optionally substituted with a substituent selected from the group consisting of fluoro, —CN, —OR26, —NR27aR27b, and —C(═O)NR27aR27b;
wherein
R24, R25a, R25b, R26, R27a, and R27b are each independently selected from the group consisting of hydrogen; C1-4alkyl optionally substituted with a substituent selected from the group consisting of fluoro and —C(═O)NR28aR28b; and C2-4alkyl substituted with a substituent selected from the group consisting of —OR29 and —NR28aR28b; wherein
R28a, R28b and R29 are each independently selected from the group consisting of hydrogen; C1-4alkyl; and C-linked 4- to 7-membered non-aromatic heterocyclyl containing at least one nitrogen, oxygen or sulfur atom; or
(h) -L2-R3 is selected from the group consisting of
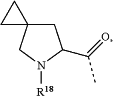 wherein R18 is hydrogen;
or a pharmaceutically acceptable salt or a solvate thereof.
|