| CPC C07D 498/04 (2013.01) [A61K 9/0014 (2013.01); A61K 9/0048 (2013.01); A61K 9/0056 (2013.01); A61K 9/008 (2013.01); A61K 9/06 (2013.01); A61K 9/2054 (2013.01); C07D 471/04 (2013.01); C07D 471/14 (2013.01); C07D 487/04 (2013.01); C07B 2200/05 (2013.01)] | 21 Claims |
|
1. A compound of Formula (XIII):
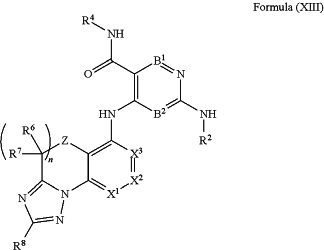 or a pharmaceutically acceptable salt, tautomer, or solvate thereof, wherein:
Z is —NR10—, —O—, —S—, —S(═O)—, or —SO2—;
X1 is CR11 or N;
X2 is CR11 or N;
X3 is CR11 or N;
B1 is CR12a and B2 is CR12b;
or B1 is N and B2 is CR12b;
or B1 is CR12a and B2 is N;
R2 is a Ring B that is an unsubstituted or substituted phenyl, unsubstituted or substituted monocyclic 6-membered heteroaryl, or unsubstituted or substituted monocyclic 5-membered heteroaryl, wherein if Ring B is substituted then Ring B is substituted with q instances of R13;
or R2 is —C(═O)R14, —C(═O)NR14R15, or —C(═O)OR14;
R4 is hydrogen, C1-C4 alkyl, or C1-C4 deuteroalkyl;
R6 is hydrogen, deuterium, halogen, C1-C4 alkyl, or C1-C4 deuteroalkyl;
R7 is hydrogen, deuterium, halogen, C1-C4 alkyl, or C1-C4 deuteroalkyl; or
or one R6 and one R7 attached to the same carbon atom are taken together with the carbon atom to which they are attached to form —(C═O)— or C3-C4 cycloalkylidenyl;
R8 is hydrogen, halogen, unsubstituted or substituted C1-C6 alkyl, unsubstituted or substituted C1-C6 deuteroalkyl, unsubstituted or substituted carbocyclyl, unsubstituted or substituted heterocyclyl, —CN, —OH, —OR17, —C(═O)R16, —CO2R16, —C(═O)N(R16)2, —N(R16)2, —NR16C(═O)R17, —SR16;
wherein the substituted C1-C6 alkyl or substituted C1-C6 deuteroalkyl is substituted with one or more substituents independently selected from the group consisting of halogen, —CN, —NH2, —OH, —NH(CH3), —N(CH3)2, —OCH3, —OCHF2, and —OCF3; and
wherein the substituted carbocyclyl or substituted heterocyclyl is substituted with one or more substituents independently selected from the group consisting of halogen, —CN, —NH2, —OH, —NH(CH3), —N(CH3)2, —CH3, —CH2CH3, —CHF2, —CF3, —OCH3, —OCHF2, and —OCF3;
R10 is hydrogen, C1-C6 alkyl, C1-C6 deuteroalkyl, C1-C6 fluoroalkyl, C3-C6 cycloalkyl, or monocyclic heterocyclyl;
each R11 is independently hydrogen, halogen, C1-C6 alkyl, C1-C6 fluoroalkyl, —CN, —OH, —OR17, or —N(R16)2;
R12a is hydrogen, halogen, C1-C4 alkyl, C1-C4 fluoroalkyl, or —CN;
R12b is hydrogen, halogen, C1-C4 alkyl, C1-C4 fluoroalkyl, or —CN;
each R13 is independently halogen, unsubstituted or substituted C1-C6 alkyl, unsubstituted or substituted carbocyclyl, unsubstituted or substituted heterocyclyl, —CN, —OH, —OR17, —C(═O)R16, —CO2R16, —C(═O)N(R16)2, —N(R16)2, —NR16C(═O)R17, —SO2R17, or —SO2N(R16)2;
wherein each substituted C1-C6 alkyl is independently substituted with one or more substituents independently selected from the group consisting of halogen, —CN, —NH2, —OH, —NH(CH3), —N(CH3)2, —OCH3, —OCHF2, and —OCF3; and
wherein each substituted carbocyclyl and substituted heterocyclyl is independently substituted with one or more substituents independently selected from the group consisting of halogen, —CN, —NH2, —OH, —NH(CH3), —N(CH3)2, —CH3, —CH2CH3, —CHF2, —CF3, —OCH3, —OCHF2, and —OCF3;
R14 is hydrogen, unsubstituted or substituted C1-C6 alkyl, C1-C6 deuteroalkyl, unsubstituted or substituted monocyclic carbocyclyl, unsubstituted or substituted bicyclic carbocyclyl, unsubstituted or substituted monocyclic heterocyclyl, or unsubstituted or substituted bicyclic heterocyclyl;
wherein the substituted C1-C6 alkyl is substituted with one or more substituents independently selected from the group consisting of halogen, —CN, —NH2, —OH, —NH(CH3), —N(CH3)2, —OCH3, —OCHF2, and —OCF3; and
wherein the substituted carbocyclyl or substituted heterocyclyl is substituted with one or more substituents independently selected from the group consisting of halogen, —CN, —NH2, —OH, —NH(CH3), —N(CH3)2, —CH3, —CH2CH3, —CHF2, —CF3, —OCH3, —OCHF2, and —OCF3;
R15 is hydrogen, C1-C6 alkyl, or C1-C6 fluoroalkyl;
each R16 is independently hydrogen, C1-C6 alkyl, C1-C6 fluoroalkyl, C1-C6 heteroalkyl, C3-C7 cycloalkyl, or monocyclic 3- to 8-membered heterocycloalkyl;
or any two R16 on the same nitrogen atom are taken together with the nitrogen atom to which they are attached to independently form a 4- to 6-membered heterocycloalkyl;
each R17 is independently C1-C6 alkyl, C1-C6 fluoroalkyl, C1-C6 heteroalkyl, C3-C7 cycloalkyl, or monocyclic 3- to 8-membered heterocycloalkyl;
n is 1; and
q is 1 or 2.
|
|
17. A pharmaceutical composition comprising a pharmaceutically acceptable excipient and the compound of claim 1, or a pharmaceutically acceptable salt, tautomer, or solvate thereof.
|