| CPC C07D 471/04 (2013.01) | 9 Claims |
|
1. A process of preparing (R)-1-((7-cyano-2-(3′-((3-(((R)-3-hydroxypyrrolidin-1-yl)methyl)-1,7-naphthyridin-8-yl)amino)-2,2′-dimethyl-[1,1′-biphenyl]-3-yl)benzo[d]oxazol-5-yl)methyl)pyrrolidine-3-carboxylic acid, or a salt thereof, comprising:
reacting a compound of formula III-5:
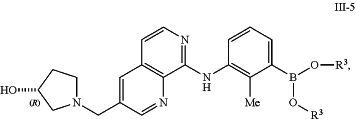 or a salt thereof, with a compound of formula IV-1:
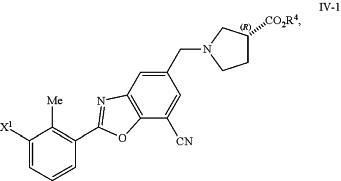 or a salt thereof, in the presence of a Suzuki catalyst and a base to form a compound of formula IV-2:
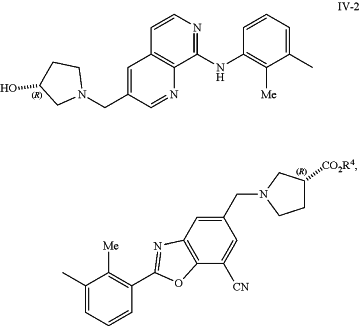 or a salt thereof, wherein:
each R3 is independently selected from H and C1-6 alkyl; or
each R3 together form an C2-3 alkylene linker, which is optionally substituted by 1, 2, 3, or 4 independently selected C1-4 alkyl groups; and
R4 is C1-6 alkyl; and
X1 is halo.
|