| CPC C07D 215/38 (2013.01) [A61P 31/14 (2018.01); C07D 401/12 (2013.01); C07D 405/12 (2013.01)] | 12 Claims |
|
1. A method for treating a patient with a RNA virus infection caused by a RNA virus belonging to group IV or V of the Baltimore classification, the RNA virus infection caused by a RNA virus belonging to a group IV or V of the Baltimore classification being chosen among a RSV viral infection, a Chikungunya viral infection, a Dengue viral infection, and an Influenza viral infection, the method comprising:
administering a therapeutically effective amount of a compound of formula (I) or a pharmaceutically acceptable salt thereof to the patient
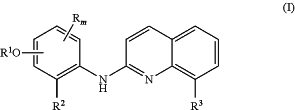 wherein
m is 0, 1 or 2,
R3 represents a chlorine atom or a hydrogen atom,
R represents a (C1-C4)alkyl group, a (C3-C6)cycloalkyl group, a halogen atom, a (C1-C5)alkoxy group, a —SO2—NRaRb group, a —SO3H group, a —OH group, a —O—SO2—ORc group or a —O—P(═O)—(ORc)(ORd) group,
R1 represents
(i) a CF3 group,
(ii) a (C1-C10)alkyl group, one carbon atom of the (C1-C10)alkyl group being optionally replaced by an oxygen atom and the (C1-C10)alkyl group being optionally substituted by one or more of a —CF3 group, halogen atom, pyridyl group, phenyl group, (C3-C6)cycloalkyl group, (C3-C6)heterocycloalkyl group or hydroxy group,
(iii) a (C3-C6)cycloalkyl group or a (C3-C6)heterocycloalkyl group, each of which is optionally substituted by one or two group(s) independently selected from a (C1-C2)alkyl group or a fluorine atom, or
(iv) a phenyl group or a naphthyl group, each of which is optionally substituted by one or two group(s) independently selected from a (C1-C4)alkyl group, a halogen atom, a —COOR′ group, a (C1-C5)alkoxy group, a —SO2—NRaRb group, a —SO3H group, a —OH group, a —O—SO2—ORc group or a —O—P(═O)—(ORc)(ORd) group,
R2 represents a (C1-C10)alkyl group, a (C3-C6)cycloalkyl or a (C3-C6)heterocycloalkyl group, the (C3-C6)cycloalkyl or (C3-C6)heterocycloalkyl groups being optionally substituted by one or two group(s) independently selected from a (C1-C2)alkyl group or a fluorine atom,
the OR1 group is in para or meta position on the phenyl with respect to the —NH— group,
R′, Ra and Rb independently represent a hydrogen atom, a (C1-C5)alkyl group or a (C3-C6)cycloalkyl group, and
Rc and Rd independently represent a hydrogen atom, Li, Na, K, N(Ra)4 or a benzyl group.
|